User:Student 56/Notebook/Biology 210 at AU
Zebrafish Observations Feb 25-3,2016
The data in the tables linked below show the raw data from day 1 through 14 of the life of the fish during our light experiment.
Setting Up Zebrafish Experiment Feb 18, 2016
The zebrafish in this experiment were used to study environmental effects on embryogenesis. The environment - and alternations to the environment - affects developing zebrafish embryos because they are extremely sensitive to the conditions that surround them.
After reading some literature about the effect of light on zebrafish development, our hypothesis was that the fish that were exposed to darkness/minimal light would have a higher mortality rate and would be smaller, and the fish that were exposed to constant light would have a higher rate of physical deformities and would exhibit different swimming patterns because of the effect on their eyes.
Experimental Setup:
3 plates with 24 wells in each one were set up with a fish egg in each well, and was filled with 2mL of dear park water. One plate was the control, which was exposed to normal levels of light and dark. Another plate was the experimental dark plate, which was wrapped in tinfoil and stuck under a cardboard box to limit sunlight exposure. The final plate was the experimental light plate, which was placed under a lamp to signify constant light.
Two days later they were fed a few drops of paramecium.
February 18, 2016 16S Sequence Analysis
The PCR that was done on Transect 4 did not work, so instead the information attained from this part of the lab was taken from Trevor Gruen in the BIO 210 Section 3 Spring 2016 who also was assigned Transect 4. Sequence is used to determine the genus of the species by comparing the gel that I take to a known gel to see if the markers line up. Whichever markers are the most similar indicates which species it is. Sequence analysis corresponds to colony morphology, gram stain, and motility studies because it is relating one type of bacteria to its known members. As the different samples were taken from the agar plate, it was known that the bacteria came from colonies. These colonies held bacteria that could be used in a gram stain in order to be looked under a microscope. The shape and motility could be characterized. Using a dichotomous key, the species of the bacteria could be guessed, but the official genus species could be named using the genotype instead of just looking at the phenotype.
Once the DNA sequence was obtained, the nucleotide sequence was put into BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi)to identify the bacteria species. The bacteria species he found was Pseudomonas putida, which is often found in soil or water environments and showed up as being 97% identical.
Because the PCR did not work, the picture attached is of a gel one of the other transects did in Section 7 of BIO lab.
The sequence was: NNNNNNNNNNNNNNNNGNNATGCAGTCGAGCGGATGAGAAGAGCTTGCTCTTCGATTCAGCGGCGGACGGGTGAGTAATG CCTAGGAATCTGCCTGGTAGTGGGGGACAACGTTTCGAAAGGAACGCTAATACCGCATACGTCCTACGGGAGAAAGCAGG GGACCTTCGGGCCTTGCGCTATCAGATGAGCCTAGGTCGGATTAGCTAGTTGGTGGGGTAATGGCTCACCAAGGCGACGA TCCGTAACTGGTCTGAGAGGATGATCAGTCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGGG AATATTGGACAATGGGCGAAAGCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGTCTTCGGATTGTAAAGCACTTTAAG TTGGGAGGAAGGGCAGTAAGCGAATACCTTGCTGTTTTGACGTTACCGACAGAATAAGCACCGGCTAACTCTGTGCCAGC AGCCGCGGTAATACAGAGGGTGCAAGCGTTAATCGGAATTACTGGGCGTAAAGCGCGCGTAGGTGGTTTGTTAAGTTGAA TGTGAAAGCCCCGGGCTCAACCTGGGAACTGCATCCAAAACTGGCAAGCTAGAGTACGGTAGAGGGTGGGTGGAATTTCC TGTGTAGCGGTGAAATGCGTAGATATAGGAAGGAACACCAGTGGCGAAGGCGACCACCTGGACTGATACTGACACTGAGG TGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTCCACGCCGTANCGATGTCAACTAGCCGTTGGAATCCT TGAGATTTTAGTGGCGCAGCTACGCATTAAGTTGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCANTGAATTGAC GGGGGCCCGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGANAACCTTACCNNCCTTGACATGCAGANAAC TTTCTAGAGATAGATTGGTGCCTTCNGGAACTCTGACACNNTGCTGCATGGCTGTCGTCAGCTCGTGTCNTGAGATGTTG GGNNAGTCCCGTANNAGCGCAACCCNTGTCCNTANTTACCAGCNCGTTATGGTGGGNACNNNTANNNNACTNCN
February 11, 2016 Invertebrates
Purpose
This lab was meant to have us used a dichotomous key to identify invertebrates found in our transect from the Berlese Funnel set up in the previous lab. It helped us understand which germ tissues gave rise to which organ types/systems. We were able to describe the differences in mechanisms of motility between flatworms, roundworms, and annelids. We defined and gave examples of acoelomates, pseudocoelomates, and coelomates. We learned how to recognize whether an animal has radial, bilateral or no symmetry. We described the differences in digestive system between flatworms, roundworms, and annelids, and defined deuterostome and protostome.
Introduction
Invertebrates were the first animals to evolve complex structures and organ systems. The simplest invertebrates are the sponges that belong to the phylum Porifera. Cells do not form true tissues, but they do have a strong association with one another. Sponges tend to be stationary with their cells oriented relative to the environment in the same direction. Sponges and invertebrates like them do not have any kind of symmetry. Cnidaria and Ctenophora are among the most primitive of these invertebrates with radial symmetry. Bilateral symmetry evolved next and it consists of a head, tail, and right and left sides.
Bilateral symmetry allowed for the development of internal organ/tissue orgaization by forming the three germ layers of tissue: ectoderm, endoderm, and mesoderm. The Platyhelminthes comprise a primitive phylum of bilateral worms with solid bodies. They are acoelomates because their simple digestive system lacks a fluid filled cavity (coelom) that separates the digestive tract from the outer body wall.
Materials and Methods
First, the acoelomate, Planaria was observed with the dissecting scope. They type of movement they use was observed and the cross section of the whole mount of it was observed with a microscope. The nematodes were then observed and a cross section slide of their pseudocoelomate structure was observed as well. The coelomate Annelida was then observed.
Organisms from each of the five major classes: arachnida, diplopoda, chilopoda, insect, and crustacea were observed, and each example was then classified correctly before moving on to the Berlese Funnel.
Last week the Berlese Funnel was set up. First 25 mL of the 50:50 ethanol/water solution was poured into the 50 mL conical tube. A piece of the screening material was placed into the bottom of the funnel, and the funnel was set up on a ring stand so that was held into the tube with the ethanol. The base was then parafilmed so the ethanol would not evaporate. A 40 watt lamp was then placed above the funnel with the incandescent bulb about 1-2 inches from the top of the leaf litter. Everything was then covered with foil and left on the bench.
The Funnel was carefully broken down and the top 10-15 mL were poured into a petri dish, and the remaining liquid was poured into a second petri dish. Both were examined under the dissecting mircoscope and all Arthropoda invertebrates observed were classified using a key online. Five invertebrates were identified, measured, and recorded in a table.
Groups of vertebrates were then looked at in the textbook and five were identified that might inhabit the transect.
Data
| Organism (phylum and class) | Length in mm | Number in sample | Description of Organism |
| 1. Arthropoda Insecta | 4 mm | 2 | Plant Lice |
| 2. Arthropoda Insecta | 2 mm | 1 | A Louse |
| 3. Arthropoda Insecta | 1 mm | 2 | A Termite |
| 4. Arthoropoda Insecta | 3 mm | 1 | A fly |
| 5. Arthropoda Insecta | 2 mm | 4 | A Louse |
The largest organism were the two Plant Lice and the smallest were the Louse. The Louse were also the most common organisms in the leaf litter around our transect. The two samples from the Berlese Funnel were very different. The top sample had no organisms in it, and the bottom sample had all of our organisms in it.
Five organisms that might inhabit our transect are the Black Squirrel (Chordata, Mammalia, Rodentia, Sciuridae, Sciurus, Sciurus, S. carolinensis), chipmunk (Chordata, Mammalia, Rodentia, Sciuromorpha, Sciuridae, Marmotini, Tamiina, Taminas), white tailed dear (Chordata, Mammalia, Artiodactyla, Cervidae, Capreolinae, Odocoileus, O. virginianus) (there have been many sightings around that section of campus in the early morning mostly in the spring), Cardinals (Chordata, Aves, Passeriformes, Passeri, Cardinalidae), and American Tree Sparrows (Chordata, Aves, Passeriformes, Embericidae, Spizelloides, S. arborea).
There are many trees, and shrubs for all the animals to eat the leaves and seeds of respectively. The plants in the transect can provide homes for all the species except the deer. The humans that live around the transect could leave food particles that could serve as sustenance for some of these species.
Drawing of the food web:
Pictures
February 4, 2016 Plants and Fungi
Purpose
This lab was geared toward giving us examples of unique characteristics that plants evolved throughout evolution. It also helped us learn how to identify and describe the differences in fungi and plants. This lab allowed us to distinguish between angiosperms and bryophytes while we gave specific examples of these. Finally, we were taught the alternation of generations in bryophytes and angiosperms and were able to identify the structure and functions of the different reproductive parts of a flower.
Introduction
It is thought that land plants evolved from green algae and that the earliest plants resembled the Bryophytes of modern day. They are mossy like plants that lived in places that weren't necessarily around water. This presented a new set of challenges that they had to adapt with: rapidly fluctuating land temperatures, access to water, access to nutrients and grasses, and interactions between male and female gametes.
Fungi are decomposers that are highly necessary for life to exist on Earth. When they "eat" things, they release CO2 into the air and nitrogen back into the soil for other organisms to use. Some fungi are unicellular, but most are filamentous and create large, highly structured shapes.
Materials & Methods
For this lab, tree Ziploc bags were obtained and leaf litter was collected from our transect. An area of soft soil and dead leaves was collected and put into a bag, this would then be used for the Berlese funnel for collecting invertebrates. Then, five plants were taken from the transect to show a diversity of plant life in the transect. These samples were placed into plastic bags and pictures were taken of where they were collected from in our transect. Seeds and flowers were also collected and put into the third plastic bag.
Back in the lab, the moss was observed and was compared to the lily plant stem. The leaves of the moss were then examined using a dissection microscope. A lily flower was then dissected to identify the different parts of the reproductive structures the plant has. Next, the seeds from our transect were then dissected and shown under the dissecting microscope.
A Berlese Funnel was then set up for our lab the next week. First 25 mL of the 50:50 ethanol/water solution was poured into the 50 mL conical tube. A piece of the screening material was placed into the bottom of the funnel, and the funnel was set up on a ring stand so that was held into the tube with the ethanol. The base was then parafilmed so the ethanol would not evaporate. A 40 watt lamp was then placed above the funnel with the incandescent bulb about 1-2 inches from the top of the leaf litter. Everything was then covered with foil and left on the bench until the next lab period.
Data
Attached to this link is the Plant Characterization table
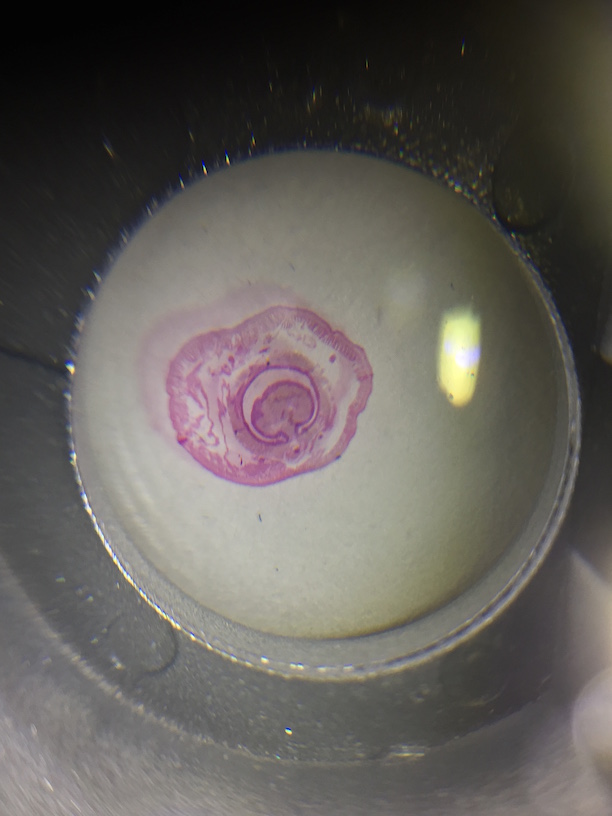
The plants were all taken in the upper part of our transect. There are not a lot of plants around our transect, but the plants that are there are large trees or bushes. There is not a lot of grass or small vegetation. There was only one species of tree with leaves still on it because of the cold weather, but these leaves were very waxy and could clearly stand to survive in the elements. This was the only plant with leaves on it. The other species did not have any live leaves on it, only dead ones around it. There was only one type of "seed" that we could find in our transect, and that was the acorn seen in the pictures portion. A cross section was cut and was also put under the microscope to see what kinds of structures it had.
Photos
January 28, 2016 Microbiology
Our hay infusion had changed from the previous week. The water was much darker and the mixture as a whole was more compact. There was much less leaf litter sticking out of the water and taking up space in the container. The mixture also didn't smell as much as it had the previous week, which was surprising. There was still a separation in the layers of the mixture, with a definite line between the bottom middle and top parts of the water.
100-fold Serial Dilutions Results
Dilution Agar Type # Colonies on Plate Conversion Factor Colonies/mL
10-3 Nutrient 1040 x 10^3 1,040,000
10-5 Nutrient 60 x 10^5 6,000,000
10-7 Nutrient 2 x 10^7 20,000,000
10-9 Nutrient 3 x 10^9 3,000,000,000
10-3 Nutrient + tet 121 x 10^3 121,000
10-5 Nutrient + tet 1 x 10^5 100,000
10-7 Nutrient + tet 1 x 10^7 10,000,000
10-9 Nutrient + tet 0 x 10^9 0
The biggest difference in the plates with and without tetracycline was the ones with the tetracycline had much less growth than the ones without tetracycline. There were two or three species of bacteria/fungi that were unaffected by the tetracycline.
To do the Gram Stain, a loop was put over a flame to sterilize and then had time to cool so that the bacteria was not killed in the heat. The loop was then used to scrape up a tiny amount of growth from the surface of the agar. The bacteria was then mixed into a drop of water on the slide. All slides were labeled with their respective dilutions and weather tetracycline was present or absent. The slide was then passed through a flame to get all the water to evaporate. The slides were then placed on a staining tray and they were covered with crystal violet for one minute. The slides were then rinsed and then were covered with Gram's iodine mordant for one minute. The slides where then rinsed again and covered a third time with 95% alcohol for 10-20 seconds. The slides were then rinsed and covered with safranin stain for 20-30 seconds. The slides were then rinsed a final time with water. The excess water was carefully blotted with a kimwipe and the slide was then left to air dry. The slides where then observed under the microscope at 40x and 100x.
2 PCR tubes were then labeled with our transect number, colony identifier, and the group number. Then, 20 microL of primer/water mixture was added to the labeled PCR tubes. The primer/water mixture was then mixed to dissolve the PCR bead at the bottom of the tube. Using a sterile toothpick, a small amount of bacterial colony was picked up and mixed into the PCR tubes. The toothpick was then discarded and the cap was placed on the tube. The tube was then placed into the PCR machine and was left overnight.
Pictures
January 21, 2016 Protists and Algae
Purpose During this lab we identified different types of Protists and Algae in our hay infusions that were made the week prior. This was helpful because it allowed us to work with a dichotomous key and learn to identify and make observations on moving organisms. We also learned more about our transect by looking at and identifying the types of organisms that live there.
Materials and Methods First, we made observations about our hay infusion to see how it changed from when we originally made it. Then we made wet mounts for microscopic observation from three different niches in the hay infusion (one sample from the bottom of the jar, one from the middle, and one from the top). We then prepared and plated serial dilutions to be used the next week in the Microbiology Lab.
To make the serial dilutions four tubes filled with 10 mLs of sterile broth were labeled with 10^-2, 10^-4, 10^-6, and 10^-8 respectively. Four nutrient agar and four nutrient agar plus tetracycline plates were also labeled 10^-3, 10^-5, 10^-7, and 10^-9 respectively for both tetracycline positive and negative plates.
The hay infusion was then swirled with the lid on to mix up all the organisms. 100 microL was then added from the hay infusion to the 10 mLs of broth in the tube labeled 10^-2. The tube was then mixed thoroughly. 100 mLs of the broth from tube 10^-2 was then added to the 10^-4 tube and so on until all the dilutions were made.
The dilutions were then plated by pipetting 100 microLs from the 10^-2 tube onto the nutrient agar plate labled 10^-3. the sample was then spread carefully so that it was even across the plate. This was repeated with the 10^-4 dilution on the 10^-5 plates and so on. The agar plates were then placed on the rack with the agar side up.
Data
Hay Infusion Observations - smells mucky, much like a pond or a mud pool - brown color with leaves sticking out of the water - layer of soil on the bottom - defined layers between the top, middle, and bottom - mold growing on the top but no green shoots
Organisms on the Top of the Hay Infusion
- Peranema: cell elongated, colorless, with a broad, rounded edges. Highly plastic when stationary, often appeared to vibrate
- Amoeba: bloblike, small and creeped along not very fast. Had a single disc-shaped nucleus
- Actinosphaerium: Spherically shaped with radiating "spines"
Organisms at in the Middle of the Hay Infusion
- Colpidium: small body, oval shaped, with small mouth; fast swimmer
- Peranema: elongated, colorless cell with a broad, rounded posterior when moving
- Pelomyxa: Large creeping organism that moves using lots of small colored things (small nuclei)
- Oedogonium: Algae where the chloroplast forms a reticulated network extending from pole to pole of each cell
Organisms at the Bottom of the Hay Infusion
- Peranema: elongated, colorless cell with a broad, rounded posterior when moving
- Chlamydomonas: Cell is oval-shaped, fast moving, two observed flagella, colorless
- Chilomonas: Cell is elongated with a narrowed posterior, moves fast, colorless, two observed flagella
Pictures of the organisms
January 13,2016 20x20 Transect
Introduction Transect Description My group was assigned to transect #4. This transect consisted of a man-made pond that is surrounded by large rocks to add to the aesthetic of the pond. There are also several statues and signs that are around the pond periodically that were put there by AU staff. There are several small trees that are around the pond that give it shade, but that also deposit leaves all over the ground. The ground around the pond was coated in leaves and debris. There were not debris in the pond itself because there was a covering over it made of sticks and mesh. Behind the transect is a residence hall, which provides much artificial lighting to be able to see in the dark.
Biotic Components
-Water
-Trees
-Small Shrub-like Plants
Abiotic Components
-Rocks
-Soil
-Dead Leaves