User:Megan Stocking/Notebook/Biology 210 at: Difference between revisions
No edit summary |
No edit summary |
||
| Line 97: | Line 97: | ||
Introduction | Introduction | ||
Prokaryotes belong to the Domains Bacteria and Archaea. Prokaryotes are the phylogenetic desendants of the most primitive unicellular organisms. Prokaryotes do not contain membrane-bound nuclei, or membrane-bound organelles. Despite their simplistic morphology, prokaryotic cells do feature cell walls enclosing its cellular plasma membranes. Bacterial cell walls are made up on a complex polysaccharide made of amino acids called peptidoglycan. Peptidoglycan gives bacterial cell walls strength and firmness. Prokaryotic cells of organisms belonging to the Domain Archaea can be distinguished from bacterial cells because the compound peptidoglycan is exclusive to bacterial cell walls. The colonies analyzed in this lab were composed of prokaryotic bacterial cells, which meant that if cell walls were present, peptidoglycan compounds would be present. In order to further analyze the types of bacterial cells that grew on the agar plates, a gram stain was able to be introduced, a dyeing system used by biologists to distinguish two types of of cell walls. For gram-positive walls, a gram stain causes cells to look purple due to rentention of crystal violet dye, and for gram-negative, cells will look pink. Most cells with a positive gram stain feature a plasma membrane with extensive peptidoglycan (Freeman, et al, 2014). Cells with a negative gram stain usually have a plasma membrane enclosed by a gelatinous layer containing less peptodoglycan and an outer phospholipid bilayer (Freeman, et al, 2014). | Prokaryotes belong to the Domains Bacteria and Archaea. Prokaryotes are the phylogenetic desendants of the most primitive unicellular organisms. Prokaryotes do not contain membrane-bound nuclei, or membrane-bound organelles. Despite their simplistic morphology, prokaryotic cells do feature cell walls enclosing its cellular plasma membranes. Bacterial cell walls are made up on a complex polysaccharide made of amino acids called peptidoglycan. Peptidoglycan gives bacterial cell walls strength and firmness. Prokaryotic cells of organisms belonging to the Domain Archaea can be distinguished from bacterial cells because the compound peptidoglycan is exclusive to bacterial cell walls. The colonies analyzed in this lab were composed of prokaryotic bacterial cells, which meant that if cell walls were present, peptidoglycan compounds would be present. In order to further analyze the types of bacterial cells that grew on the agar plates, a gram stain was able to be introduced, a dyeing system used by biologists to distinguish two types of of cell walls. For gram-positive walls, a gram stain causes cells to look purple due to rentention of crystal violet dye, and for gram-negative, cells will look pink. Most cells with a positive gram stain feature a plasma membrane with extensive peptidoglycan (Freeman, et al, 2014). Cells with a negative gram stain usually have a plasma membrane enclosed by a gelatinous layer containing less peptodoglycan and an outer phospholipid bilayer (Freeman, et al, 2014). | ||
Antibiotics are molecules that kill bacteria or stop them from growing; tetracycline is an antibiotic. Recent research has uncovered that antibiotic-resistance of bacterial strains are linked to the advantage that bacteria possess to grow as biofilms- dense colonia forms of bacteria enmeshed in a polysaccharide-rich matrix (Freeman, et al, 2014). Biofilms help shield bacteria from antibiotics. | Antibiotics are molecules that kill bacteria or stop them from growing; tetracycline is an antibiotic. Recent research has uncovered that antibiotic-resistance of bacterial strains are linked to the advantage that bacteria possess to grow as biofilms- dense colonia forms of bacteria enmeshed in a polysaccharide-rich matrix (Freeman, et al, 2014). Biofilms help shield bacteria from antibiotics. When antibiotics are administered to treat a bacterial growth or infection, they work by interrupting peptidoglycan synthesis; for example, in a gram-positive bacteria which contain extensive peptidoglycan, an administered penncilin attacks the exisiting cell wall and disrupts more peptidoglycan from synthesizing (Pearson, 2010). The peptidoglycan within gram-negative cells is much harder for antibiotics to penetrate, and therefore have much lower susceptibility to antibiotics; when antibiotics are administered to gram-negative bacterial cells, their cell walls are not completely destroyed (Pearson, 2010). | ||
Revision as of 09:54, 9 July 2014
07/07/2014 Biological Life on Campus: Observing A Mini Marsh, One of Many Niches at AU
Introduction
Biological life at American University is ecologically diverse. The biodiversity observed on campus is attributable to various types of ecosystems present throughout its entire physical landscape. Each ecosystem, or transect, is characterized by both its topographic and geographic features, as well as its biotic and abiotic components. The unique ecosystem surveyed for this lab belonged to transect #1, or the “mini marsh.” A marsh is a type of wetland, usually defined by terrestrially low-lying geographies, frequent incidence of flooding or presence of watershed, and grassy, herbaceous vegetation. The mini marsh is located on the Northeastern side of Main campus, on the lawn situated where busy Massachusetts Avenue perpendicularly meets the Kogod School of Business. The precise location of the transect is within closer proximity to the building entrance than the street, as it lies at the foot of the hill that proceeds outward from the building entrance, designating the beginning of the lawn of the lawn, or, at the innermost interior of the lawn with respect to the street on the other side. The transect area was not planar, but slightly sloped due to the area’s contiguity with more steeply sloping of parts of the surrounding landscape. To make a more informed hypothesis, some additional background information was gleaned from the American University Sustainability Homepage. Of the many initiatives set by AU to routinely accomplish “low-impact, low-maintenance, low-resource-use,” those of note included the use of native plants, preventing soil erosion with ground cover and dense planting on sloped areas, integrative pest management, and reducing harmful runoff (American University, 2014). Furthermore, "xeriscaping," a modern mechanism for manual landscaping that seeks to accomplish water-efficient vegetation by way of elemental design, has also been a proponent of AU across campus ecosystems. Based on this information, the following presumptions were made: 1)the initial construct of the marsh's design was made with respect to organism selection based on abiotic and biotic limitations, and 2) all of the expected ecological components would be present of an inland marsh. With those two things in mind it was hypothesized that a mutualist, efficient ecosystem would be observed of the mini marsh.
Materials and Methods
To begin the lab, access to the requisite transect was required; this was accomplished by going to the site of transect #1 on the Eastern side of Main campus. Adequate time was utilized to observe the biological life inhabiting transect #1, as well as the inorganic constituents contributing to the niche. A sterile 50mL conical tube was used to take a soil and ground vegetation sample representative of the ground surface of transect #1.
Results
Tables and Graphs
 Figure 2: Abiotic and Biotic Components
Figure 2: Abiotic and Biotic Components
Discussion
The initial hypothesis is supported based on the analysis of the components of transect #1 for numerous reasons. Not only were the abiotic and biotic components of the mini marsh consistent with the characteristics that typically define this type of niche, microbial analysis and secondary research also indicated that the transect was consistent with AU’s initiatives by way of ease of facilitation for the interrelation of abiotic and biotic components to carry out dynamic, mutualistic functions. Elemental design attributed to the efficiency of the marsh's ecosystem. The tall, white-flower-bearing plants were identified as the evergreen shrub Mountain Laurel (Kalmia latifolia), which is a native species that blooms in July, which explained the relative abundance of flower clusters present at that time. This species is perennial and requires only partial sunlight to sustain life. It also lives in wet, dry or clay soil sustained by the species’ deep lateral roots that render the species as drought-tolerable, and it can survive on any physical gradient. Summer blooming usually results in spring-like pollination assisted by bees (Virginia Department of Conservation & Recreation, 2014). This plant is also a vital component for butterfly wildlife, as butterflies feed on the nectar produced from the flowers. The nectar in these flowers is toxic for humans and most other large animals and this characteristic makes Mountain Laurels a safe haven for butterflies to lay eggs or undergo larvae development. This is just one of the many biotic components observed in the mini marsh, but its role is exemplary in terms of contributing to carrying out specific requirements for life within the niche.
Another family inhabiting the mini marsh is the fern plant. At least three species were identified, all native plants of the region: those identified were the Evergreen Wood fern (Dryopteris intermedia), the New York fern (Thelypteris noveboracensis), and the Holly fern (Cyrtomium falcatum). The fern plants varied between 1-3 feet in height and were represented on complementary quadrants equidistant from the sewage drain. This presence of fern species are akin to the Mountain Laurel as biotic components because both populations low-maintenance and ecologically efficient with respect to the conditions of their niche. Like the Mountain Laurel, ferns too are tolerable to shade and partial sunlight and their root systems make them extremely water-efficient. These ferns have rhizome roots, which grow into horizontal root systems that act not only in water and nutrient reserve, but also provide stability to the soil during harsher climates like winter. Ferns also provide ground coverage in the mini marsh niche (Jurries, 2013). Because of the way these fern species become rooted and grow, ferns are able to grow densely, on a multitude of gradients, and by doing so, the ferns can do two things: effectively block sunlight from being able to reach the ground beneath to facilitate the growth of weeds, and act as a barrier to rain drops from penetrating soil to the extent possible as the first line of defense to prevent soil erosion from rain water. Additionally, ground coverage provides a habitat for small animals like squirrels and birds. The ferns and the Mountain Laurel are both evergreen, which means that they lose their leaves gradually instead of all at once, indicating that this niche has a consistent source of organic compost.
The expected vegetation was present- abundant tall grasses, some herbaceous plan types, and there were also cattails. At first, the observation of cattails was concerning since they are known as an invasive species. However, given the proportion of common cattail (Typha latifolia) to those of the grass species present, it seemed that the cattail population was well within a controlled quantity. Three species of grass were identified and as native species, these were Deer Tongue grass (Dichanthelium clandestium), Little Bluestem grass (Schizachyrium scoparium), Longhair Sedge (Carex comosa). Deer Tongue grass tolerates high levels of aluminum in soil making it an ideal biotic component for filtration of storm water, and it sustains itself in acidic and infertile soils. Deer Tongue grass serves as a food source for insects including grasshoppers, caterpillars, and beetle species, as well as small mammalian herbivores such as rabbits. The seeds of Deer Tongue Grass are a food source for Sparrows (Virginia Department of Conservation and Recreation, 2013). Cattails and grasses act in concert to provide a water filtration system that maintains water quality, prevents polluted runoff that can impact the larger biosphere, and reduces the incidence of soil erosion caused by storm water or pollutant waste. Both plants absorb large amounts of phosphorous, a type of inorganic material that, if it were not filtered by the marsh vegetation, could be disposed through the sewage system and enter a local watershed where it may cause a toxic environmental condition called algal bloom (Jurries, 2003). Grasses bearing seeds are a food source for birds and small mammals, as well as the decayed grasses contribute to revegetation in the ecosystem’s biotic cycle. Cattails provide bird species with raw cotton product from which to make habitats. Lastly, it was observed, and subsequently understood through further investigation that microorganisms within the mini marsh niche play a tremendous role in its ecological success, contributing to the completion of biogeochemical and biotic cycling that involves both nonliving and living components as work (Carnegie Mellon, 2014). Plankton and algae that live in the soil of the marsh are two of many microorganisms that also play a particular role in the water filtration. Both protists types partially remove soluble forms of phosphorus and ammonia from storm water. Algae consumes nutrients and converts them into biomass which it remitted to the marsh’s soil for uptake by vegetation when needed in order to sustain growth and life (Jurries, 2003).
Because of fitness trade-offs and its subsequent effect on an organism’s tendency to adapt to a limited set of physical conditions, and because every organism has a specific range of tolerance for those conditions, or abiotic factors (Freeman, et al, 2014). When added that the ability of a species to persist in an area is limited by biotic factors, which relies on the living interrelationships within the niche, it is plausible to conclude that the mini marsh’s composition of native species is largely contributory to the efficiency and compatibility of the marsh’s ecosystem.
References
Freeman, Scott. Allison, Lizabeth. Quillin, Kim. 2014. “Biological Science.” Fifth Edition. Illinois: Pearson Education. Pages 169-185.
“Biogeochemical Systems.” 2014. Carnegie Mellon University Open Learning Institute. (Accessed July 6, 2014) <https://oli.cmu.edu/ >
Jurries, Dennis.January, 2003. "Biofilters (Bioswales, Vegetative Buffers, & Constructed Wetlands for Storm Water Drainage and Pollution Removal)." State of Oregon: Department of Environmental Quality. Accessed July 6, 2014. <http://www.deq.state.or.us/wq/stormwater/docs/nwr/biofilters.pdf>
Virginia National Heritage Program and Database Search. 2014. The Virginia Department of Conservation and Recreation. Accessed July 6, 2014. <http://www.dcr.virginia.gov/natural_heritage/dbsearchtool.shtml>
July 7, 2014 Microbial Ekaryotes: An Underappreciated and Vital Necessity
Introduction
All living things are made up of cells and a cell is classified by its internal structural complexity and by the way it obtains energy. Prokaryotic cells are unicellular, lack a true nucleus, and do not contain membrane-bound organelles. Eukaryotes may either be unicellular or multicellular, but do contain membrane bound nuclei as well as membrane-bound organelles. Living things are classified phylogenetically, based on common ancestral species and the descendant relationships that have resulted from evolution by natural selection. Prokaryotes are classified into either of two Domains- Domain Bacteria and Domain Archaea. Eukaryotes on the other hand, are classified into one of seven Domains. It was hypothesized that that the organisms inhabiting the niche at the top of the Hay Infusion Culture were likely going to be photosynthesizing organisms; therefore, it was highly probable that algae was going to inhabit the top niche, in order to access light to perform photosynthesis. The bottom niche was likely to be inhabited by aquatic microorganisms, such as plankton, and species acting as decomposers and consumers who are not carrying out photosynthesis.
Materials and Methods
A 50mL representative soil sample was collected from transect #1, American University's "mini marsh." A Hay Infusion Culture was made using a 11.6 grams of the soil sample, combined with 500mLs of water and 0.1 gram dried milk into a plastic jar. The contents were then mixed gently for 10 seconds. The lid was then removed and the open jar containing the mixture was stored in incubation for a period of two days inside of the laboratory. Following the incubation period, an entirely independent observable ecosystem was produced within the jar. Initial observations were made about the culture’s detailed appearance, smell, and speculative questions were noted. Four wet mounts were prepared from the culture. Two wet mounts contained samples taken from the top of the jar; the other two contained samples taken from the bottom of the jar. Of the two samples taken from the top of the culture, one slide contained a suspended sample collected from the surface of the liquid , and the other slide contained a sample from an attached growth adhered to jar wall. All four wet mounts were examined under compound microscopes until one organism had been successfully identified from each. A depiction for each organism was made, and measurements of the identified microorganisms were recorded.
Results
 Figure 1: Hay Infusion Culture, Transect #1
Figure 1: Hay Infusion Culture, Transect #1
The Hay Infusion Culture was murky with sedimentary particles at the bottom of the jar. The smell was odorous, like a strong mold akin to rotton food.
 Figure 2: Hay Infusion Culture, Top Niche
Figure 2: Hay Infusion Culture, Top Niche
The first two wet mounts were taken from this niche; the first was taken from the growth along the jar wall, and the second was taken from the liquid.
 Figure 3: Hay Infusion Culture, Bottom Niche
Figure 3: Hay Infusion Culture, Bottom Niche
The second two wet mounts were made from this niche, each sample was taken from a different area at the bottom of the jar.
 Figure 4
Figure 4
 Figure 5
Figure 5
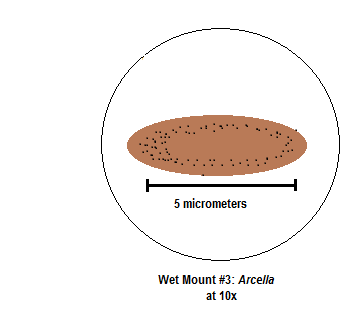 Figure 6
Figure 6
 Figure 7
Figure 7
Discussion
Two organisms were identified and observed that belonged to the niche present at the top of the Hay Infusion Culture; these organisms were Gonium and Pandorina. The Gonium colony observed consisted of approximately 8 cells. The second organism identified was Pandorina, a colonial form of green algae. Both organisms identified in the first [top] niche are very similar species; both are identified as members of the Chlorophyta group which are the green algae, and contain chlorophyll a and b unobscured by other accessory pigments like beta –carotene or other carotenoids (Bellinger, 2004). Pandorina is slightly more complex than Gonium, as both algae come from the volvocine series. Both Gonium and Pandorina were performing photosynthesis, acting as autotrophic producers in the ecosystem of the top layer niche of the Hay Infusion Culture. The primary differences between these two organisms were exhibited in their cytological presentations. The structural complexities that differentiate Pandorina from Gonium as a similar species were visible in the globular, motile colonial form observed of Pandorina, compared to that of the flattened, filamentous, branched colonial form of Gonium which was not observed to be motile at the time of study. The microorganisms identified from the bottom of the Hay Infusion Culture were both protists. The first microorganism identified was Arcella. By way of the dichotomous key, the microorganism was classified based on the observational determinants of colorlessness, motility exhibited by sliding slowly or floating without apparent motion, not possessing spherical shape, possessing a constant shape, and possessing a flattened test or shell without embedded or attached material in additional to exhibiting pale to brown color (Ward’s, 2002). Arcella is a zooplankton, the non-photosynthetic planktonic species, and relates to the Amoebozoa lineage. Arcella potentially serves as a bioindicator of the ecosystem in the Hay Infusion Culture, due to the species’ ability to survive in nutrient-depleted environments; it is also known as being an “oligotrophic bioindicator” for this reason (Hunt, 2001). Arcella species are heterotrophic decomposers and feed on detritus (Freeman, et al, 2014). The mechanism by which feeding is done Is phagocytosis, accomplished by Arcella’s flexible membrane that lacks cell walls and its finger-like projections , pseudopodia (Freeman, et al, 2014). The second microorganism identified from the bottom niche or the Hay Infusion Culture ecosystem was Stentor. The Stentor was classified according to the system, concluded by the observable characteristics of a ciliate possessing dark bluish-green color. Stentor is related to the ciliates and belongs to the Alveolata linkage. The Stentor was very motile and at least one of two present flagella was observed; cilia were visualized along the broader cellular end. The Stentor is a unique component to consider the biodiversity of the Hay Infusion culture compared with the other microorganisms because Stentor is a symbiont. In particular, Stentor and types of green algae have had symbiotic relationships, where Stentor consumes and gains nutrients from the algae, but the algae remains living and in turn sustains life using the Stentor’s metabolic waste (Methacton, 2013). Gonium is an example living organism that meets all five criteria for living organisms. It acquires and uses energy by way of respiration through photosynthesis. It is made up of cells, the fundamental units of life. Gonium contains DNA because it contains chloroplasts which contain DNA. Gonium replicates by isogamy, and its population within the algae continues to evolve, as Gonium is a descended species from a simpler unicellular organism, and other algae species, including Pandorina observed have proceeded as similar species. It appeared that the community within the Hay Infusion Culture was affected largely by the disturbance of nutrient availability, causing a disturbance in the metabolic balance of the organisms, and the respective biomass associated with the ecosystem's net productivity. It would be expected that in two months, the ecosystem would restore balance through use of the mechanisms that were observed in the identified organisms, including symbiotic relations, and continuance of biotic cycling.
References
"Protists." October 16, 2013: Methacton.org. (Accessed 7/6/14) <http://www.methacton.org/cms/lib/PA01000176/Centricity/Domain/345/protist%20book.pdf>
Freeman, Scott. Allison, Lizabeth. Quillin, Kim. 2014. “Biological Science.” Fifth Edition. Illinois: Pearson Education. Pages 2-500.
Hunt, Kendall. "Zooplankton." (2004) Kendall/Hunt Publishing Company: United States. Accessed 7/6/14. <http://books.google.com/books?id=FNyUfNu_PVMC&pg=PA223&lpg=PA223&dq=what+is+arcella+protist+in+ecosystem&source=bl&ots=G6_0tHOrOQ&sig=nqfHEL-a7Uk5EbYG5JuWnNF3BUM&hl=en&sa=X&ei=U925U4uhBpSuyASA8YLICg&ved=0CEsQ6AEwBQ#v=onepage&q&f=false>
July 9, 2014
Introduction
Prokaryotes belong to the Domains Bacteria and Archaea. Prokaryotes are the phylogenetic desendants of the most primitive unicellular organisms. Prokaryotes do not contain membrane-bound nuclei, or membrane-bound organelles. Despite their simplistic morphology, prokaryotic cells do feature cell walls enclosing its cellular plasma membranes. Bacterial cell walls are made up on a complex polysaccharide made of amino acids called peptidoglycan. Peptidoglycan gives bacterial cell walls strength and firmness. Prokaryotic cells of organisms belonging to the Domain Archaea can be distinguished from bacterial cells because the compound peptidoglycan is exclusive to bacterial cell walls. The colonies analyzed in this lab were composed of prokaryotic bacterial cells, which meant that if cell walls were present, peptidoglycan compounds would be present. In order to further analyze the types of bacterial cells that grew on the agar plates, a gram stain was able to be introduced, a dyeing system used by biologists to distinguish two types of of cell walls. For gram-positive walls, a gram stain causes cells to look purple due to rentention of crystal violet dye, and for gram-negative, cells will look pink. Most cells with a positive gram stain feature a plasma membrane with extensive peptidoglycan (Freeman, et al, 2014). Cells with a negative gram stain usually have a plasma membrane enclosed by a gelatinous layer containing less peptodoglycan and an outer phospholipid bilayer (Freeman, et al, 2014). Antibiotics are molecules that kill bacteria or stop them from growing; tetracycline is an antibiotic. Recent research has uncovered that antibiotic-resistance of bacterial strains are linked to the advantage that bacteria possess to grow as biofilms- dense colonia forms of bacteria enmeshed in a polysaccharide-rich matrix (Freeman, et al, 2014). Biofilms help shield bacteria from antibiotics. When antibiotics are administered to treat a bacterial growth or infection, they work by interrupting peptidoglycan synthesis; for example, in a gram-positive bacteria which contain extensive peptidoglycan, an administered penncilin attacks the exisiting cell wall and disrupts more peptidoglycan from synthesizing (Pearson, 2010). The peptidoglycan within gram-negative cells is much harder for antibiotics to penetrate, and therefore have much lower susceptibility to antibiotics; when antibiotics are administered to gram-negative bacterial cells, their cell walls are not completely destroyed (Pearson, 2010).
