User:Mary Mendoza/Notebook/CHEM 581: Experimental Chemistry I/2014/09/05: Difference between revisions
From OpenWetWare
Mary Mendoza (talk | contribs) |
Mary Mendoza (talk | contribs) |
||
| Line 26: | Line 26: | ||
===Preparation of Sample Pans with Hermetic Lids=== | ===Preparation of Sample Pans with Hermetic Lids=== | ||
*Films that were suspended in malachite green solutions ([[User:Mary Mendoza/Notebook/CHEM 581: Experimental Chemistry I/2014/08/29|08/29/2014]]) were individually taken out from their scintillating vials. | *Films that were suspended in malachite green solutions ([[User:Mary Mendoza/Notebook/CHEM 581: Experimental Chemistry I/2014/08/29|08/29/2014]]) were individually taken out from their scintillating vials. | ||
* Incised approximate 3 mg portions from each film; | * Incised approximate 3 mg portions from each film; each film was transferred to a Tzero pan and sealed with a hermetic lid. | ||
===Conditions for the DSC=== | ===Conditions for the DSC=== | ||
Revision as of 11:12, 12 September 2014
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
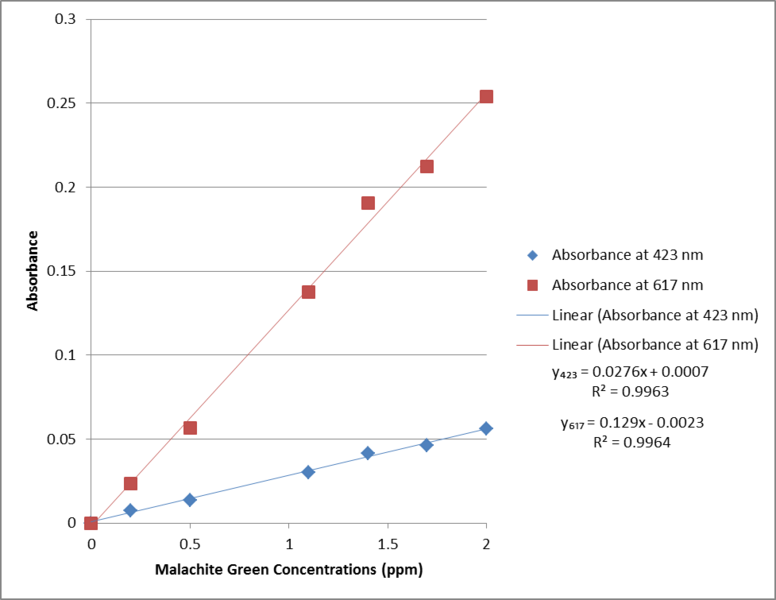
Calibration Curves for Malachite Green Stock Solutions
  Cross-linking and Neutralizing Batch 3 Films
Batch 4 Film Synthesis
Differential Scanning Calorimetry (DSC) of Sample Films with absorbed Malachite Green (MG)Preparation of Sample Pans with Hermetic Lids
Conditions for the DSCFourier Transform Infrared Spectroscopy (FTIR) of MG sample films
 
| |