User:Madison Hayes/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
'''February, | '''February, 10, 2014''' | ||
Introduction: | Introduction: | ||
The title of this experiment is [[Identifying Algae and Protists.]] The purpose of this experiment was to practice identifying groups of organisms using a dichotomous key and then put the practice to use identifying organisms in the hay infusion culture. The end of the experiment involved diluting the hay infusion culture and applying the dilutions to agar plates to set up for an experiment next week. | |||
The title of this experiment is [[Identifying Algae and Protists.]] The purpose of this experiment was to practice identifying groups of organisms using a dichotomous key and then put the practice to use identifying organisms in the hay infusion culture. The purpose is to identify the 6 organisms in the hay infusion sample created from soil and plant matter from transect five. The end of the experiment involved diluting the hay infusion culture and applying the dilutions to agar plates to set up for an experiment next week. | |||
Materials and Method: | Materials and Method: | ||
The following materials were utilized in the experiment: a two page dichotomous key, a sample of known organisms, microscope, slides, covers, transfer pipettes,protozoa, hay infusion culture (created last week). In order to prepare the dilutions for next week the following materials were needed: hay infusion culture, four tubes, 10 mL of water, micropipeters and tips, spreader, 4 agar plates without tetacycline, and three with tetracycline. | The following materials were utilized in the experiment: a two page dichotomous key, a sample of known organisms, microscope, slides, covers, transfer pipettes,protozoa, hay infusion culture (created last week). In order to prepare the dilutions for next week the following materials were needed: hay infusion culture, four tubes, 10 mL of water, micropipeters and tips, spreader, 4 agar plates without tetacycline, and three with tetracycline. | ||
| Line 18: | Line 20: | ||
9. Take 100 microliters from the tube and add it to the next tube labeled 10^-4. Mix the tube. | 9. Take 100 microliters from the tube and add it to the next tube labeled 10^-4. Mix the tube. | ||
10. Repeat two more times to create 10^-6 and 10^-8 dilutions. | 10. Repeat two more times to create 10^-6 and 10^-8 dilutions. | ||
11. For the nutrient agar plates, take 100 microliters in | 11. For the nutrient agar plates, take 100 microliters in 10^-2 and place on the surface of the nutrient agar labeled 10^-3. Repeat with the tetracycline plate labeled 10^-3. Carefully use a spreader to spread the sample around the plate. Make sure to use two seperate spreaders: one for the normal nutrient agar plates and one for the tetracycline plates. | ||
12. Repeat the exact procedure for the remaining three tubes. The weakest dilution will only be placed on a nutrient agar plate not a tetracycline plate. | |||
13. Allow the plates to sit at room temperature for a week. | |||
| Line 54: | Line 58: | ||
Observations: | Observations: | ||
Had the hay infusion been allowed to sit for another two months, I would hypothesize that the evidence of organisms would be more prevalent to the naked eye as the populations of organisms would be much greater. Another outcome could be that the organisms run out of nutrients and the organisms die. The slides would display unmoving organisms. | Had the hay infusion been allowed to sit for another two months, I would hypothesize that the evidence of organisms would be more prevalent to the naked eye as the populations of organisms would be much greater. Another outcome could be that the organisms run out of nutrients and the organisms die. The slides would display unmoving organisms. | ||
Conclusion: | Conclusion: | ||
---- | |||
'''January 31, 2014''' | '''January 31, 2014''' | ||
Revision as of 19:33, 9 February 2014
February, 10, 2014
Introduction:
The title of this experiment is Identifying Algae and Protists. The purpose of this experiment was to practice identifying groups of organisms using a dichotomous key and then put the practice to use identifying organisms in the hay infusion culture. The purpose is to identify the 6 organisms in the hay infusion sample created from soil and plant matter from transect five. The end of the experiment involved diluting the hay infusion culture and applying the dilutions to agar plates to set up for an experiment next week.
Materials and Method:
The following materials were utilized in the experiment: a two page dichotomous key, a sample of known organisms, microscope, slides, covers, transfer pipettes,protozoa, hay infusion culture (created last week). In order to prepare the dilutions for next week the following materials were needed: hay infusion culture, four tubes, 10 mL of water, micropipeters and tips, spreader, 4 agar plates without tetacycline, and three with tetracycline.
The procedures for the practice are as follows: 1. Carry the culture to the work station being careful not to jostle the environment in the jar. 2. Record your observations of the jar. 3. Take a sample from the jar and place a small drop on a slide and place a slide cover over it. 4. Characterize at least three different organisms from the slide. Draw pictures of each and measure the size. See if they can be identified with the key. 5. Repeat the steps with a sample from another area in the jar. 6. Obtain 4 tubes with 10 mL of water. Label the tubes with 2,4,6, and 8. 7. Find 4 nutrient agar plates and 3 nutrient agar plates plus tetracycline. Label the plates and add initials. 8. Shake the hay infusion mixture with the lid on and take 100 microliters and add it to the tube labeled 10^-2. Mix tube. 9. Take 100 microliters from the tube and add it to the next tube labeled 10^-4. Mix the tube. 10. Repeat two more times to create 10^-6 and 10^-8 dilutions. 11. For the nutrient agar plates, take 100 microliters in 10^-2 and place on the surface of the nutrient agar labeled 10^-3. Repeat with the tetracycline plate labeled 10^-3. Carefully use a spreader to spread the sample around the plate. Make sure to use two seperate spreaders: one for the normal nutrient agar plates and one for the tetracycline plates. 12. Repeat the exact procedure for the remaining three tubes. The weakest dilution will only be placed on a nutrient agar plate not a tetracycline plate. 13. Allow the plates to sit at room temperature for a week.
Observations and Data:
The following describes the hay infusion culture unperturbed by motion. The dirt previously in the jar collected at the bottom. The water could be described as a murky clear mixed with some opaque white clouds. Some chunks of dirt floated on the top of the water instead of settling at the bottom. Pieces of the grass that had previously been in the infusion could not be seen; either the grass disintegrated or was buried among the dirt at the bottom of the jar. The jar had no particular smell that was noticeable.
The first niche from which the sample was taken was the top of the water. The second niche from which the sample was taken was from the bottom near the dirt. The samples were not taken from near any particular plant matter, but if it was the organisms could differ because organisms prefer different environments. Plant matter would offer different nutrients and a different environment to organisms leading to a different sample.
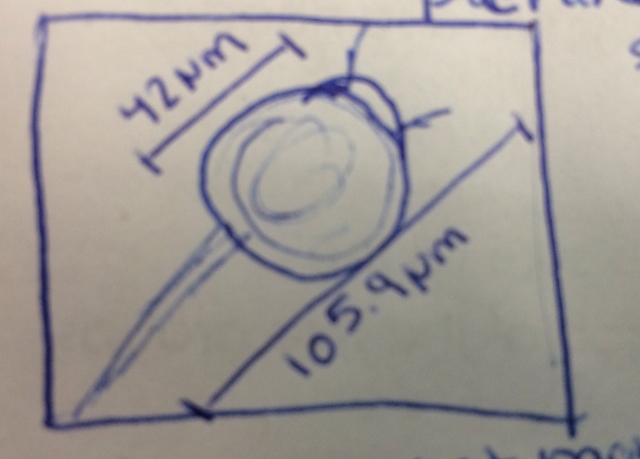
Niche 1--
Organism 1-Peranema sp
Organism 2- Colpidium sp
Organism 3- Pandorna
Niche 2--
Organism 1- Pandorina
Organism 2- Paramecium Bursaria
Organism 3- Chilomanas
Observations: Had the hay infusion been allowed to sit for another two months, I would hypothesize that the evidence of organisms would be more prevalent to the naked eye as the populations of organisms would be much greater. Another outcome could be that the organisms run out of nutrients and the organisms die. The slides would display unmoving organisms.
Conclusion:
January 31, 2014
Lab 1
Introduction: This lab was titled "Biological Life at AU". This lab was made up of two parts. The purpose of part 1 was to understand the cellular evolution of the volvacine line. The purpose of part two was to analyze the transects that will be assigned to us for the whole semester.
Procedure:
For part one the procedures are as follows: 1)Prepare slides of Chlamydomonas(make sure it is living) and analyze under microscope. 2)Add protoslo if necessary to properly view the alga. 3) Locate the conspicuous chloroplast and the the pyrenoid. 4)Repeat the steps examining volvox and gonium under a microscope. 5)For each sample record the data in the chart
For Part two the procedures were as follows: 1)Travel to assigned transect. 2)Describe the general characteristics of the transect. 3)List the abiotic and biotic factors of the transect 4)Using a 50mL conical tube take a soil and ground vegetation sample. 5)Weigh 10 to 12 grams of the soil and place in a plastic jar with 500 mL of deerpark water. 6)Add .1 grams dried milk and mix gently for 10 seconds. 7)Place in the back of the room with the top off.
Observations and Data
Part 1
Though evolution does not always lead to more complex organisms, that is the case with the volvacine line. The least complex of the three alga was the Chlamydomonas. The line evolutionarily advances as to gonium and then volvox. The colonies increase as from chlamydomonas to gonium and then to volvox.
Part 2
For this experiment transect 5 was analyzed. The transect is located in front of Hurst. The plot encompasses the following biotic factors: grass, rose bushes, bugs, and weeds. Soil, stone, bench, rocks, and a stone sign were observed abiotic factors. The location is in the middle of the quad and is relatively flat. The area is of high traffic so I would not predict too many larger animals will be seen in the transect. The vegitation in general was dead or winterized. More observations will be made throughout the semester.
Good start. Take care not to just re-write the directions from the protocol but to include real information. There could have been more information given and both parts explained in more detail. Include hypotheses and discussion of the text in red from the protocol. SK