User:Madeleine Y. Bee/Notebook/CHEM-571 2013F/2013/09/03: Difference between revisions
From OpenWetWare
No edit summary |
|||
| (8 intermediate revisions by the same user not shown) | |||
| Line 9: | Line 9: | ||
==September 3, 2013== | ==September 3, 2013== | ||
===Objective=== | ===Objective=== | ||
Execute | Execute [http://openwetware.org/wiki/User:Matt_Hartings/Notebook/AU_Biomaterials_Design_Lab/2013/09/03 procedure] for | ||
making several dilutions of adenosine and inosine solutions, measuring adsorption spectra, constructing calibration curves, performing statistical analyses, and determining concentration of 'unknowns' using calibration curves. | making several dilutions of adenosine and inosine solutions, measuring adsorption spectra, constructing calibration curves, performing statistical analyses, and determining concentration of 'unknowns' using calibration curves. | ||
===Procedure Prep=== | ===Procedure Prep=== | ||
====Stock Solutions and Dilutions==== | =====Stock Solutions and Dilutions===== | ||
Adenosine MW: 267.24g/mol<br.> | Adenosine MW: 267.24g/mol<br.> | ||
Inosine MW: 268.2g/mol<br.> | Inosine MW: 268.2g/mol<br.> | ||
| Line 24: | Line 24: | ||
{|style="width:700px" | {|style="width:700px" | ||
|<u>Adenosine solution concentrations (M)</u> | |<u>Adenosine solution concentrations (M)</u> | ||
|<u>Volume stock solution added ( | |<u>Volume stock solution added (μL)</u> | ||
|<u>Inosine solution concentrations (M)</u> | |<u>Inosine solution concentrations (M)</u> | ||
|<u>Volume of stock solution added ( | |<u>Volume of stock solution added (μL)</u> | ||
|- | |- | ||
|3.00x10<sup>-5</sup> | |3.00x10<sup>-5</sup> | ||
|3. | |3.66 | ||
|4.80x10<sup>-5</sup> | |4.80x10<sup>-5</sup> | ||
|6. | |6.08 | ||
|- | |- | ||
|2.50x10<sup>-5</sup> | |2.50x10<sup>-5</sup> | ||
|3. | |3.05 | ||
|4.00x10<sup>-5</sup> | |4.00x10<sup>-5</sup> | ||
|5. | |5.06 | ||
|- | |- | ||
|2.00x10<sup>-5</sup> | |2.00x10<sup>-5</sup> | ||
|2. | |2.44 | ||
|3.20x10<sup>-5</sup> | |3.20x10<sup>-5</sup> | ||
|4. | |4.05 | ||
|- | |- | ||
|1.50x10<sup>-5</sup> | |1.50x10<sup>-5</sup> | ||
|1. | |1.83 | ||
|2.40x10<sup>-5</sup> | |2.40x10<sup>-5</sup> | ||
|3. | |3.04 | ||
|- | |- | ||
|1.00x10<sup>-5</sup> | |1.00x10<sup>-5</sup> | ||
|1. | |1.22 | ||
|1.60x10<sup>-5</sup> | |1.60x10<sup>-5</sup> | ||
|2. | |2.03 | ||
|- | |- | ||
|0.50x10<sup>-5</sup> | |0.50x10<sup>-5</sup> | ||
| | |0.610 | ||
|0.80x10<sup>-5</sup> | |0.80x10<sup>-5</sup> | ||
| | |0.101 | ||
|- | |- | ||
|RANDOM: 0.25x10<sup>-5</sup> | |RANDOM: 0.25x10<sup>-5</sup> | ||
| | |0.305 | ||
|RANDOM: 0.40x10<sup>-5</sup> | |RANDOM: 0.40x10<sup>-5</sup> | ||
| | |0.506 | ||
|} | |} | ||
===Data=== | ===Data=== | ||
====Absorption Spectra==== | =====Absorption Spectra===== | ||
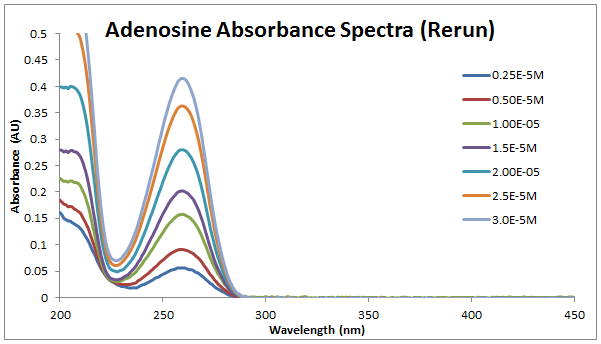
[[Image: | [[Image:2013_0904_adenosine_spectra_(rerun).PNG]]<br.> | ||
All peaks at 259nm. | All peaks at 259nm. | ||
| Line 73: | Line 73: | ||
Determine the confidence interval for 90% and 95% confidence.<br.> | Determine the confidence interval for 90% and 95% confidence.<br.> | ||
Determine if any data can be ruled out using a Q-test.<br.> | Determine if any data can be ruled out using a Q-test.<br.> | ||
====Calibration Curves==== | =====Calibration Curves===== | ||
[[Image:2013_0903_adenosine_calibration.PNG]] | [[Image:2013_0903_adenosine_calibration.PNG]] | ||
=== | ===Measurement Notes=== | ||
* | *Microliter amounts were measured with a micropipet | ||
* | *50mL volumetric flasks were used to make stock solutions of both adenosine and inosine | ||
Revision as of 11:44, 4 September 2013
 Experimental Biological Chemistry: Fall 2013 Experimental Biological Chemistry: Fall 2013
|
<html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | ||||||||||||||||||||||||||||||||
September 3, 2013ObjectiveExecute procedure for making several dilutions of adenosine and inosine solutions, measuring adsorption spectra, constructing calibration curves, performing statistical analyses, and determining concentration of 'unknowns' using calibration curves. Procedure PrepStock Solutions and DilutionsAdenosine MW: 267.24g/mol<br.> Inosine MW: 268.2g/mol<br.> <br.> Original stock solutions:<br.> Adenosine: (from 0.1091g in 50mL volumetric flask) 0.0082M<br.> Inosine: (from 0.1060g in 50mL volumetric flask) 0.0079M<br.> <br.> Dilutions to make, each with total volumes of 1mL:<br.>
DataAbsorption SpectraCalculations and AnalysisDetermine the standard deviation for your data points.<br.> Determine the confidence interval for 90% and 95% confidence.<br.> Determine if any data can be ruled out using a Q-test.<br.> Calibration CurvesMeasurement Notes
| |||||||||||||||||||||||||||||||||