User:Madeleine Naish/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
'''March 1, 2015: Zebrafish Lab Report Introduction''' | |||
The development of life is a fascinating, curious area of study in biology, as it is such a temperamental and fragile process—and yet an obviously successful one. How a life begins and grows into a functioning organism differs across domains and species, and yet much can be learned from a single, specific study on one creature’s journey toward being. It is this idea that prompts this experiment, on the development of zebrafish embryos in an environment with an extreme factor—in this case high salinity. | |||
Zebrafish have been used before in many developmental studies. Their clear embryos allow for easy visualization of the changes occurring to the organisms (Chen et. al., 2008). A simple change in a factor of their environment can result in a very revealing study. And, indeed, previous studies have been conducted on the effect of high salinity in the water in which the fish live. Resulting data has shown that high salinity combined with high temperature can cause reduced oxygen consumption and nitrogen excretion (Uliano et. al., 2010); in other studies signs of increased salt tolerance connected with advancing development were seen, as well as zebrafish not surviving past hatching (Sawant et. al., 2001). Because salt is a normal component of a zebrafish’s environment, analyzing slight differences in its concentration is an interesting study to pursue. Its outcome can be applied to other organisms, as all organisms must develop, be affected by their environment, and try to survive as these two things occur at once. | |||
The hypothesis of this experiment is that increased salt will inhibit the growth of the zebrafish. This entails that more salt in the environment will lead to stunted growth and reduced embryonic development, resulting in deformities. | |||
''Bibliography'' | |||
Chen, Yau-Hung, Yi-Hui Huang, Chi-Chung Wen, Yun-Hsin Wang, Wei-Li Chen, Li-Chao Chen, and Huey-Jen Tsay. "Movement Disorder and Neuromuscular Change in Zebrafish Embryos after Exposure to Caffeine." ''Neurotoxicology and Teratology'' 30 (2008): 440-47. Web. 1 Mar. 2015. | |||
Sawant, M. S., S. Zhang, and L. Li. "Effect of Salinity on Development of Zebrafish, Brachydanio Rerio."''Current Science'' 81.10 (2001): 1347-350. Web. 1 Mar. 2015. | |||
Uliano, E., M. Cataldi, F. Carella, O. Migliaccio, D. Iaccarino, and C. Agnisola. "Effects of Acute Changes in Salinity and Temperature on Routine Metabolism and Nitrogen Excretion in Gambusia (Gambusia Affinis) and Zebrafish (Danio Rerio)." ''Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology'' 157.3 (2010): 283-90. Web. 1 Mar. 2015. | |||
MN | |||
'''February 25, 2015: Bacteria Information Update''' | '''February 25, 2015: Bacteria Information Update''' | ||
Revision as of 16:09, 1 March 2015
March 1, 2015: Zebrafish Lab Report Introduction
The development of life is a fascinating, curious area of study in biology, as it is such a temperamental and fragile process—and yet an obviously successful one. How a life begins and grows into a functioning organism differs across domains and species, and yet much can be learned from a single, specific study on one creature’s journey toward being. It is this idea that prompts this experiment, on the development of zebrafish embryos in an environment with an extreme factor—in this case high salinity.
Zebrafish have been used before in many developmental studies. Their clear embryos allow for easy visualization of the changes occurring to the organisms (Chen et. al., 2008). A simple change in a factor of their environment can result in a very revealing study. And, indeed, previous studies have been conducted on the effect of high salinity in the water in which the fish live. Resulting data has shown that high salinity combined with high temperature can cause reduced oxygen consumption and nitrogen excretion (Uliano et. al., 2010); in other studies signs of increased salt tolerance connected with advancing development were seen, as well as zebrafish not surviving past hatching (Sawant et. al., 2001). Because salt is a normal component of a zebrafish’s environment, analyzing slight differences in its concentration is an interesting study to pursue. Its outcome can be applied to other organisms, as all organisms must develop, be affected by their environment, and try to survive as these two things occur at once.
The hypothesis of this experiment is that increased salt will inhibit the growth of the zebrafish. This entails that more salt in the environment will lead to stunted growth and reduced embryonic development, resulting in deformities.
Bibliography
Chen, Yau-Hung, Yi-Hui Huang, Chi-Chung Wen, Yun-Hsin Wang, Wei-Li Chen, Li-Chao Chen, and Huey-Jen Tsay. "Movement Disorder and Neuromuscular Change in Zebrafish Embryos after Exposure to Caffeine." Neurotoxicology and Teratology 30 (2008): 440-47. Web. 1 Mar. 2015.
Sawant, M. S., S. Zhang, and L. Li. "Effect of Salinity on Development of Zebrafish, Brachydanio Rerio."Current Science 81.10 (2001): 1347-350. Web. 1 Mar. 2015.
Uliano, E., M. Cataldi, F. Carella, O. Migliaccio, D. Iaccarino, and C. Agnisola. "Effects of Acute Changes in Salinity and Temperature on Routine Metabolism and Nitrogen Excretion in Gambusia (Gambusia Affinis) and Zebrafish (Danio Rerio)." Comparative Biochemistry and Physiology Part A: Molecular & Integrative Physiology 157.3 (2010): 283-90. Web. 1 Mar. 2015.
MN
February 25, 2015: Bacteria Information Update
The MB# tube labels for our bacteria were 11 and 12.
Sequences
11: NNNNNNNNNNNNNNNNTGCAGTCGAGCGGANGANGGGAGCTTGCTCCTGGATTCAGCGGCGGACGGGTGAGTNNNGNCTAGGAATCTGCCTGGTAGTGGGGGACAACGTTTCGAAAGGAACGCTAA TACCGCATACGTCCTACGGGAGAAAGCAGGGGACCTTCGGGCCTTGCGCTATCAGATGAGCCTAGGTCGGATTAGCTTGTTGGTGAGGTAATGGCTCACCAAGGCGACGATCCGTAACTGGTCTGA GAGGATGATCAGTCACACTGGAACTGAGACACGGTCCAGACTCCTACGGGAGGCAGCAGTGGGGAATATTGGACAATGGGCGAAAGCCTGATCCAGCCATGCCGCGTGTGTGAAGAAGGTCTTCGG ATTGTAAAGCACTTTAAGTTGGGAGGAAGGGTTGTAGATTAATACTCTGCAATTTTGACGTTACCGACAGAATAAGCACCGGCTAACTCTGTGCCAGCAGCCGCGGTAATACAGAGGGTGCAAGCG TTAATCGGAATTACTGGGCGTAAAGCGCGCGTAGGTGGTTTGTTAAGTTGGATGTGAAAGCCCCGGGCTCAACCTGGGAACTGCATCCAAAACTGGCAAGCTAGAGTACGGTAGAGGGTGGTGGAAT TTCCTGTGTAGCGGTGAAATGCGTAGATATAGGAAGGAACACCAGTGGCGAAGGCGACCACCTGGACTGATACTGACACTGAGGTGCGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGT CCACGCCGTNNCGATGTCAACTAGCCGTTGGAATCCTTGAGATTTTAGTGGCGCAGCTAACGCATTAAGTTGACCGCCTGGGGAGTACGGCCGCAAGGTTAAAACTCAAATGAATTGACGGGGGCC CGCACAAGCGGTGGAGCATGTGGTTTAATTCGAAGCAACGCGAANAANCTTANCNNNCTTGACATGCANANAACTTTCCANANATGGATTGGNGCCTTCGGNAACTCTGACNCAGNG CTGCATGNNNGNTCGTCAGCTCGTGTCGTGAGANNNTGNNTNAGTCCCGNTANCNANCGNNNNNCNNGTCCNTANTNNNCAGCNNNTTNTGNNN
12: NNNNNNNNNNTNNNNNNNNNNNAGTCGNNCGGCANCNNGATCTAGCTTGCTAGATTGATGGCGAGTGGCGAACGGGTGAGTAATACATCGGAACGTGCCCTGTAGTGGGGGATAACTAGTCGAAAGAT TAGCTAATACCGCATACGACCTGAGGGTGAAAGTGGGGGACCGCAAGGCCTCATGCTATAGGAGCGGCCGATGTCTGATTAGCTAGTTGGTGGGGTAAAGGCCCACCAAGGCGACGATCAGTAGCTGG TCTGAGAGGACGATCAGCCACACTGGGACTGAGACACGGCCCAGACTCCTACGGGAGGCAGCAGTGGGGAATTTTGGACAATGGGCGAAAGCCTGATCCAGCAATGCCGCGTGTGTGAAGAAGGCCTT CGGGTTGTAAAGCACTTTTGTCCGGAAAGAAATGGCTCTGGTTAATACCTGGGGTCGATGACGGTACCGGAAGAATAAGGACCGGCTAACTACGTGCCAGCAGCCGCGGTAATACGTAGGGTCCAAGC GTTAATCGGAATTACTGGGCGTAAAGCGTGCGCAGGCGGTTGTGCAAGACCGATGTGAAATCCCCGAGCTTAACTTGGGAATTGCATTGGTGACTGCACGGCTAGAGTGTGTCAGAGGGGGGTAGAAT TCCACGTGTAGCAGTGAAATGCGTAGAGATGTGGAGGAATACCGATGGCGAAGGCAGCCCCCTGGGATAACACTGACGCTCATGCACGAAAGCGTGGGGAGCAAACAGGATTAGATACCCTGGTAGTC CACGCCCTAAACGATGTCAACTAGTTGTTGGGGATTCATTTCCTTAGTAACGTANCTAACGCGTGAAGTTGACCGCCTGGGGAGTACGGTCGCAAGATTAAAACTCAAAGGAATTGACGGGGACCCGC ACAAGCGGTGGATGATGTGGATTAATTCGATGCAACGCGAAAAACCTTACCTACCCTTGACATGCCACTAACGAAGCANANATGCATTANGTGCTCNAAAGAGAAAGTGGANNCNNNGCTGCATGGCT GTCGTCAGCNNCGTGTCGTGANNATGTTNGGGTTAAGTCCCCGCAACGAGCNNCANCCCTTGTCTNCTAGTTGCNNN
Top Matches
11: Pseudomonas sp. NCCP-571 gene for 16S ribosomal RNA
12: Ralstonia sp. DT 4-07 16S ribosomal RNA gene
MN
February 11, 2015: Invertebrates and Vertebrates
I. Purpose
In this experiment, the goal was to further comprehend the vast diversity of life that can be found within our transect sample, and how the different organisms present can affect one another. This time, the types of organisms studied were invertebrates and vertebrates. In order to gain more knowledge about these two types of organisms, several small procedures were completed within this experiment, all with the hypothesis that the invertebrates and vertebrates would affect each other. This hypothesis leads to the idea that the invertebrates and vertebrates in some way depend on each other as they go about sharing the same ecosystem.
II. Materials and Methods
For the first procedure, the invertebrates nematodes, in a cross sectional slide, were studied under a microscope. This cross sectional slide allowed for observation of their pseudocoelomate structure, as well as their movement.
The next procedure was more complex and required a week’s worth of time. A leaf litter sample from the transect was collected. This sample came from an area of soft soil covered with dead leaves. These dead leaves and covering plant matter were placed into a Ziploc bag, as well as a small amount of the rough top layer of dirt. All together 500g of the leaf litter were placed into the Ziploc bag. In the lab, a Berlese Funnel was then put together for this leaf litter. During the construction of this funnel, 25 mL of a 50:50 ethanol/water solution was poured into a 50 mL conical tube. Screening material was attached to the bottom of a funnel using tape to keep leaf and large matter from falling into the preservative. The leaf litter sample was carefully placed on top of the funnel; this funnel was then carefully placed in a ring stand, with its lower shoot attached to the conical tube with parafilm. This construction was placed beneath a lit 40 watt lamp, the bulb 1-2 inches from the litter. The litter was then covered with foil. The funnel construction was left like this for a week.
Once the week had passed, the funnel was broken apart and the conical tube was separated from the rest. The top 10-15 mLs of the liquid within the tube was poured into a petri dish. The remaining liquid was poured into a second dish. These dishes were studied under a microscope to find any existing invertebrates.
In the final procedure of this experiment, the marsh transect itself was analyzed for vertebrates. Five were identified and classified. These vertebrates were used to create a food web of the marsh transect.
III. Data and Observations
The study of the invertebrates revealed several different characteristics. For example, the nematodes that were studied for movement were found to sway their upper bodies from side to side in order to propel themselves forward. The study of the invertebrates found in the Berlese Funnel, however, brought forth much more variation in terms of results because of the number of different organisms that could be found in the sample. These results can be observed in Table 1.
The results in Table 1 reveal some observable patterns. The Springtail primitive was the largest organism, while the Beetle mite and the Psocid tied for the smallest. Mites of various types appear to be the most commonly found organisms in this leaf litter.
The five different vertebrates found and identified in the marsh transect are the following:
The Eastern Grey Squirrel (Sciurus carolinensis) Phylum: Chordata; Class: Mammalia; Order: Rodentia; Family: Sciuridae; Genus: Sciurus; Species: S. carolinensis
The Field Sparrow (Spizella pusilla)
Phylum: Chordata; Class: Aves; Order: Passeriformes; Family: Emberizidae; Genus: Spizella; Species: S. pusilla
The American Robin (Turdus migratorius)
Phylum: Chordata; Class: Aves; Order: Passeriformes; Family: Turdidae; Genus: Turdus; Species: T. migratorius
The Common Garter Snake (Thamnophis sirtalis)
Phylum: Chordata; Class: Reptilia; Order: Squamata; Family: Colubridae; Genus: Thamnophis; Species: T. sirtalis
The Northern Short-tailed Shrew (Blarina brevicauda)
Phylum: Chordata; Class: Mammalia; Order: Soricomorpha; Family: Soricidae; Genus: Blarina; Species: B. brevicauda
Once all of these organisms were identified, it was possible to learn more about their characteristics, such as habitat and diet. This knowledge allowed a food web of the marsh transect to be created, including not only these five vertebrates but all the organisms from the marsh transect previously studied. This food web can be seen in Figure 1.
IV. Conclusions and Future Directions
The hypothesis for this experiment proved to be correct. The vertebrates and invertebrates observed in this experiment would certainly affect each other, as would all the organisms within the marsh transect would affect one another—a standard concept of an ecosystem. These organisms affect each other in several ways. The vertebrates often seek the invertebrates as a source of food. However the invertebrates, which will often eat plant matter, allow for the plant life in the ecosystem to stay healthy by cleaning up decaying matter. The vertebrates would depend on this because seeds and fruits are also a common food source to them, and these would only be available if the nearby plant life were healthy. Indeed, the invertebrates and vertebrates, though very different physically and in terms of behavior, both have large effects on each other as they inhabit the same ecosystem. And the environment of this ecosystem, the marsh transect, must benefit the organisms living within it. Abiotic and biotic elements of the environment, such as the plants, would contribute to this environment. Analyzing the environment’s effect on the vertebrates in particular, they would benefit from the marsh’s natural environment in terms of shelter and diet. Insects such as earthworms are a common form of sustenance for several of the vertebrates, including the Field Sparrow, the American Robin and the Common Garter Snake. The many tall plants growing in the transect would provide many resources as well: the Eastern Grey Squirrel could use plant pieces for its nest, and both the birds and the snake prefer plant cover when roosting or sleeping. These plants would also create seeds that could be fed on by the organisms. The moisture of the marsh transect is also attractive to organisms such as the snake, which prefers moist environments.
All together, the group of organisms studied throughout the lab section on the transect display several concepts of ecology. These organisms, of many, many different species, all live together in an ecosystem and thus form a community despite their differing species. Carrying capacity plays a factor in the life of this community, as it is the principle that keeps the organisms from overproducing or joining together in this transect. Only a certain amount of organisms can live in a particular ecosystem, and the matters of predation and decomposition by bacteria allow this amount to stay below carrying capacity. Finally, the analysis of the food chain and the predation within this transect give a view to how trophic levels can work—as organisms move farther and farther away from the direct source of food, photosynthesizers. Because nearly all the organisms in this transect eat a photosynthesizer, the trophic level variation will not be intense as in other transects; the shrew and the snake are probably some of the organisms highest on the trophic level. Either way, many concepts of ecology can be seen in this transect, and these fundamental concepts prove the hypothesis of this experiment correct.
MN
February 4, 2015: Analyzing Transect Plants
I. Purpose
In order to visualize and understand the genotypic and phenotypic changes that a species can go through, this experiment’s analysis of several plants from different locations on the evolutionary timeline was conducted. In particular, the presence of the characteristics vascularization, specialized structures and mechanism of reproduction were analyzed and compared. The hypothesis for this experiment was that plants with more recent spots on the evolutionary timeline would feature more recent developments of these three characteristics. This means that such plants would display vascularization and flowering as a mechanism of reproduction.
II. Materials and Methods
The retrieval of plant samples from the marsh transect started off the experiment. Five samples from five different plants were obtained and placed into a Ziploc bag. These samples included a cattail, hay, a flowering bush, a butterfly bush and moss. Photos were taken of the full plants from which these samples were taken. Seeds and flowers from these plants were also included when possible.
In the lab, these five plants along with lily (Lilium) and moss (Mnium) samples were analyzed. The stem of the lily and a piece of the moss were studied for vascularization and height; then the cross section of the lily was studied to find the xylem and phloem layers within it. Five cross sections were created for each plant by cutting small pieces of their stems off and placing them on a slide with a drop of water, and then viewing them under a microscope, in order to determine the vascularization of each.
Both the moss and lily sample were then studied, for the presence of specialized structures such as cuticle and stomata, with the use of a low magnification compound scope. The leaves, if present, of each of the five plants were studied as well for shape, size and cluster arrangement. This was done by cutting off small pieces of the leaves and then placing them onto a slide with a drop of water and viewing them under a microscope. Finally, the moss, lily and plant samples were analyzed for mechanism of reproduction. The moss was viewed through the low magnification compound scope for identification of the male and female gametophytes and sporophytes, as well as the haploid and diploid parts of its life cycle. The lily sample was then studied for the presence of several parts that involve reproduction: the anther, stigma and style. The seeds of the transect plants were studied if applicable by placing small pieces of them on a slide with a drop of water and then viewing then under a microscope in order to identify them as either monocot or dicot, as well as to detect any flowers or spores present.
Some fungi samples were quickly studied as well, with the use of a dissecting microscope. They were studied to determine the type of fungi they are and which of the three divisions of a particular fungi lineage they belong to.
III. Data and Observations
Images of the five transect samples can be seen in Figures 1 through 5, below.
These five samples were analyzed for various characteristics. The results of this analysis can be seen in Table 1.
The two fungi samples that were analyzed were found to be of two different divisions from the same lineage. The black mold that was studied was found to be of the Ascomycota division, while the mushroom was of the Basidiomycota division. A common characteristic of these fungi is the Fungi sporangia, an area or enclosure where spores can form, a very important thing as this can help with reproduction. The underside of Basidiomycota sample—the mushroom—was composed of dark overlapping layers. This organism would be identified as a fungus because of the dark layers on its underside, known as basidia, which produce sexual basidiospores. A drawing of this underside can be seen in Figure 6 below.
IV. Conclusions and Future Directions
The hypothesis for this experiment proved to be correct. Of the five transect plants studied, those that were more recent on the evolutionary timeline displayed more recent developments of the characteristics of vascularization, specialized structures and mechanism of reproduction. The four most recent plants all happened to be flowering, or angiosperms, and displayed various forms of vascularization and specialized structures, with examples being seed distribution and stomata. The fifth plant was a moss, meaning it was non-vascular, reproduced through spores and only had rhizoids as a slightly specialized structure. All of these characteristics are rather undeveloped and not complex, in comparison to those displayed by the four flowering plants. This experiment could be improved in the future by using more samples that are not flowering, because the hypothesis was imperfect in that flowering is not the only means of reproduction that is a recent evolution of plants.
MN
January 28, 2015: Bacterial Microbiology and Antibiotic Resistance
I. Purpose
Though the jar of the Hay Infusion Culture was a small space, countless organisms could thrive and be found in it. Samples from the culture have been used throughout the past experiments, and in the present one. In order to further understand the complexities of bacteria, their susceptibility to antibiotics, and their overall incredible variance, this experiment of serial dilution and bacterial observation was conducted. The hypothesis for this experiment was that bacteria unexposed to antibiotic would be more populous and complex, but not of the Achaea species, as the environment is not extreme enough. This means that the bacteria unexposed to the antibiotic will be gram-positive.
II. Materials and Methods
In order to inoculate samples of prokaryotic organisms from the Hay Infusion Culture, serial dilutions were prepared and plated. Four tubes of 10 mLs sterile broth were labeled as 10-2, 10-4, 10-6 and 10-8. Then eight agar plates were retrieved: four nutrient agar and four nutrient agar plus tetracycline, the latter of which were labeled with “tet.” One plate from each group was labeled as 10-3, 10-5, 10-7 and 10-9. The Hay Infusion Culture was swirled, and then 100 µL from the culture was sampled with the use of a micropippetor set at 100 µL. These 100 µL were added to the 10 mLs of broth in the 10-2 tube, and this tube was then swirled. 100 µL from tube 10-2 were added to 10-4; this step was repeated twice more to create the 10-6 and 10-8 dilutions. 100 µL from the 10-2 tube were then pipetted and spread onto both the 10-3 agar plates—the one with tetracycline and the one without. This same process was done with the 10-4 dilution and 10-5 plates, the 10-6 dilution and 10-7 plates, and the 10-8 dilution and 10-9 plates. The plates were then closed and place side up on a rack where they stayed and incubated for a week at room temperature.
After the week had passed, the plates were retrieved. The total number of colonies on each plate were counted. They were analyzed for presence of antibiotic resistance to tetracycline. Then, four samples—two from each group—were chosen to be used for wet mounts. For the wet mount procedure, a metal loop was sterilized over a flame and then used to scrape a colony from each of the selected plates. These colony samples were mixed into a drop of water on their own separate slides. These wet mounts were then observed with a microscope in both 10X and 40X in order to see cell shape, image and motility.
For the gram stain procedure, a metal loop was once again sterilized over a flame and used to scrape colonies from the four selected plates. These colonies were mixed into drops of water on four separate slides. The four slides were then passed through the flame three times with the bacterial smear side facing up. The slides were placed on a staining tray and covered with crystal violet for one minute, then rinsed off with water. They were then covered with Gram’s iodine mordant for another minute, and washed with water once again. In order to decolorize the smears they were flooded with 95% alcohol for 10-20 seconds, and then rinsed with water. The smears were covered with safranin stain for 20-30 seconds and then rinsed with water a final time. Excess water was gently wiped off the slides with kimwipes. After air drying, the gram stains were observed with a microscope in low and high magnifications.
III. Data and Observations
The Hay Infusion Culture did not smell as strongly as before. The surface of the water had been grown over with a substance during its week of stillness. Such weekly changes are most likely due to the decaying matter inside, as well as the resulting lack of oxygen and survival and dying of different species inside the jar. The results can be seen in Figures 1 and 2.
 Figure 1: Hay Infusion Culture side view
Figure 1: Hay Infusion Culture side view
 Figure 2: Hay Infusion Culture aerial view
Figure 2: Hay Infusion Culture aerial view
The overall number of colonies found on the agar plates were much greater on the nutrient agar plates without tetracycline than on those with tetracycline. However, colonies were still found on the agar plates treated with the antibiotic. Fungi also continued to grow regardless of the presence of tetracycline, and in fact were more prevalent on the tetracycline plates. The eight agar plates can be seen in Figures 3 and 4.
 Figure 3: Nutrient Agar Plates without Tetracycline
Figure 3: Nutrient Agar Plates without Tetracycline
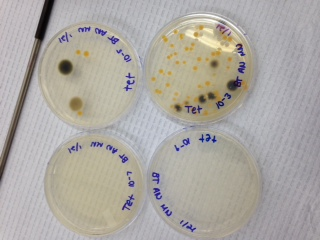 Figure 4: Nutrient Agar Plates with Tetracyline
Figure 4: Nutrient Agar Plates with Tetracyline
The colony count for all eight agar plates can be found in Table 1.
The four colonies taken from the plates for the gram stain were NA 10-3 and 10-5, as well as tet 10-3 and tet 10-5. The colony sampled from NA 10-3 was clear; the colony from NA 10-5 was only slightly darker orange. The samples from both tet plates, however, were equally bright and orange, and came from large circular colonies. The visual result of the gram stain can be seen in Figure 5.
The descriptions of the bacteria observed in the gram stain slides can be found in Table 2.
IV. Conclusions and Future Directions
The data from the colony count show a reaction to the presence of tetracycline. While colonies were present on all four nutrient agar plates without the antibiotic, colonies were found on only two of the four tet plates. This shows that the antibiotic is somewhat effective at stopping bacterial growth; however, the antibiotic resistance occurring in this experiment is also indisputable. Half of the plates failed to grow colonies, and half succeeded, allowing for a 50% success rate of the antibiotic tetracycline. It also did little to stop fungi. When tetracycline was first used as an antibiotic, it was able to overpower both gram-positive and gram-negative bacteria, as well as protozoan parasites and chlamydiae, among other things, but now antibiotic resistance of tetracycline is a large problem. When successfully inhibiting a bacteria, tetracycline will prevent a bacterial tRNA from reaching its bacterial ribosome, meaning it must move through various membranes, the number depending on whether the bacteria is gram-positive or gram-negative. Usually once this traversing through membranes is over it is the lipophilic form of the tetracycline that will do the main work on the ribosomes (Chopra and Roberts, 2001). This means that, when bacteria develop resistance, they will either cause an efflux of the antibiotic through strong use of energy or will somehow be able to protect their ribosomes. At the present, 32 gram-negative and 22 gram-positive bacteria have been found to possess tetracycline resistance genes; such bacteria can be found in pathogens and flora species (Roberts, 1996). It is clear that some of the bacteria sampled from the Hay Infusion Culture are among those resistant to the antibiotic.
The hypothesis for this experiment was thus proved to be correct, as the bacteria incubated on agar plates without the antibiotic were more populous, showing greater numbers of colonies. However, the prediction was incorrect—gram-positive bacteria were found from both groups of plates, are notably susceptible to the antibiotic, and indeed have nothing to do with whether or not they were placed on a tet plate—and the hypothesis was not really sufficiently telling of what the main purpose of this experiment was. A stronger hypothesis might have been that no bacteria would grow on the tet plates, or that some percentage of them would.
Chopra, Ian, and Marilyn Roberts. "Tetracycline Antibiotics: Mode of Action, Applications, Molecular Biology, and Epidemiology of Bacterial Resistance." Microbiology and Molecular Biology Reviews 65.2 (2001): 232-60. PubMed Central. Web. 3 Feb. 2015.
Roberts, M. C. "Tetracycline Resistance Determinants: Mechanisms of Action, Regulation of Expression, Genetic Mobility, and Distribution." FEMS Microbiology Reviews 19.1 (1996): 1-24. PubMed. Web. 3 Feb. 2015.
MN
January 21, 2015: Algae and Protists in Hay Infusion Culture
I. Purpose
In this experiment, different organisms from different areas of a hay infusion culture are observed. The purpose of these observations are to allow for a better understanding of the degree of biodiversity that can be found in ecosystems—even very small ones. The hypothesis for this experiment is that organisms from two different niches of the culture—the surface and the bottom of the jar—will themselves be very different. A prediction for this experiment is that the organisms at the bottom of the jar will be smaller.
II. Materials and Methods
The soil and plant life of the marsh transect of American University was sampled from and placed into a sterile 50 mL conical tube. 10-12 grams of the sample was placed in a plastic jar, along with 500 mLs of deerpark water and 0.1 gm dried milk. The contents of the jar were mixed gently, and then the jar’s lid was removed. The jar remained in the lab in this state for a week.
After a week, two slides were made of two samples from the transect. One sample was drawn from the surface of the water, while the other was drawn from the dark dirt at the bottom of the jar. Both samples were drawn out with plastic pipettes and placed on slides and covered with coverslips. Three different organisms were identified from each wet mount with the use of a microscope and dichotomous key.
III. Data and Observations
After a week in the jar, the hay infusion culture had a very strong, swampy smell. A white, mold-like substance had grown at the surface, and brown dirt and other substances had accumulated at the bottom of the jar.
From the sample taken from the surface of the water, three different organisms were observed:
Paramecium multimicronucleaum, a motile cell at 100 µm;
Blepharisma sp., a non-motile cell at 450 µm;
and Pandorina, a colony with seven large cells and many more small ones, measuring at 70 µm.
From the sample taken from the bottom of the jar, three other different organisms were observed:
Paramecium bursaria, a small, dark, motile cell measuring at 50-60 µm;
Amoeba proteus, a motile, dotted cell measuring at 20 µm;
and Colpidium sp., a cilia-covered cell measuring at 60 µm.
IV. Conclusions and Future Directions
This experiment revealed that a great amount of biodiversity can exist in very small ecosystems. The six organisms found in the hay infusion culture never repeated. Though the hypothesis was proved correct for this experiment, because the organisms found at the top were different from those found at the bottom, this finding did not reveal much about the variation of niches within an ecosystem because it was not specific enough, and because the organisms within the same niches were ‘different.’ Different was not sufficiently defined, which is a necessity and something that would improve any future trials of this experiment. However, the prediction that the organisms at the bottom would be smaller than those at the top was proved correct, and this is slightly more insightful. All the organisms from the bottom of the jar measured to be 20-60 µm, while those from the top grew much bigger at 70-450 µm.
One reason the organisms at the surface of the culture may have differed from those at the bottom is their differing proximity to the plant matter in the jar. Though the plant matter was generally quite mixed up in the culture, a lot of it was floating at the surface, meaning the organisms from the top would have been closer to the plant life than those at the bottom. Proximity to plant matter can change those organisms nearby because plant matter will eventually decompose, and nearby organisms can get nutrition from this process. This would be how an organism from the top niche would probably meet one of the needs of life: energy. Pandorina is an example from the top. Because this organism lives in a colony it is composed of several cells that can transmit information to each other and continue replicating to increase their number. They most likely show signs of evolution simply by being in colonies, which is complex. After two months of living in this ecosystem, however, these complex organisms might not be faring so well. So much of the existing plant matter would have decomposed, and so much of the available oxygen would have been used up without proper replacement. This would make it difficult for Pandorina, and the other organisms, to survive.
MN
January 14, 2015: Evolution observed in the Volvocine Line
I. Purpose
In order to better understand the effects of evolution over time, three different organisms from the Volvocine Line will be observed in this experiment. These members of the Volvocine Line are known to have evolved from each other, and therefore their differences and similarities can give new insight on how evolution leads to genotypic and phenotypic change. The hypothesis for this experiment is that, the more recent a member of the Volvocine Line is that is being studied, the more complex it will be. If this is true, then the more complex organisms will be found in increasingly larger colonies.
II. Materials and Methods
Slides were made for the three organisms. This required samples, taken with the use of plastic pipettes, from Chlamydomonas, Gonium and Volvox, which were placed onto slides and then covered with coverslips. Before being covered with a coverslip, a drop of protoslo was placed on Chlamydomonas for easier viewing. All three organisms were viewed under a microscope, to allow their cell number, colony size, mechanisms of motility and method of sexual reproduction to be observed.
III. Data and Obseravations
The results of the study of the members of the Volvocine Line can be seen in Table 1.
Drawings of the view of the organisms through the microscope can be seen below.
IV. Conclusions and Future Directions
The experiment revealed the more recent organisms were more complex than the older organisms. This complexity has to do with several things. First, it has to do with whether or not the cells formed a colony. Chlamydomonas, the oldest organism, does not live in colonies, whereas Gonium and Volvox were found to live in colonies. Volvox, the youngest organism, lived in the largest colony, with hundreds of cells in each. Complexity also has to do with how the cells sexually reproduced—either through isogamy or oogamy, the latter being the more complex method. Chlamydomonas, the oldest organism, once more displayed the least complexity by reproducing through isogamy, while the other two organisms reproduced through oogamy.
The results of the experiment show that the process of evolution can lead to more complexity, proving the hypothesis correct. This complexity was indeed displayed through the quality of colonies, among other things. This experiment provided a good understanding of the kind of changes evolution can lead to. However, because increased complexity is not always the result of evolution, a future experiment might observe organisms that did not grow more complex through evolution.
Niches at AU
The niche I observed was the marsh at American University. This niche is located across the street from the Katzen Arts Center, at the base of a slightly sloped hill, with part of its perimeter next to pavement. The other part is surrounded by grass, though this grass ends not too far off, with the pavement along the street. There is a manhole in the rock area, but it is otherwise a very natural niche with several types of plants. Some abiotic factors within the transect are light, coming from the sky above, water, coming from the snow melting on the ground, rock, dirt, which is the bottom layer of the transect, and the manhole. Some biotic factors are moss on the ground, grass on the ground, clovers growing in the eastern area, the butterfly bush growing in the southeastern corner, and cattails growing near the center of the transect.
File:Transect.png Transect
MN
January 12, 2015
I have successfully entered a post!
MN






















