User:Khyra A. Neal/Notebook/Chem 571/2014/10/14: Difference between revisions
From OpenWetWare
No edit summary |
No edit summary |
||
| (2 intermediate revisions by the same user not shown) | |||
| Line 28: | Line 28: | ||
** Run UV-Vis of undialyzed 0.6 g/L Lysozyme solution with Bradford reagent | ** Run UV-Vis of undialyzed 0.6 g/L Lysozyme solution with Bradford reagent | ||
[[Image: Dialyzed_Lysozyme_in_KI.jpg]] | |||
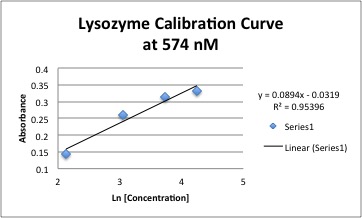
3. Calibration Curve for undialyzed Lysozyme by Bradford Analysis | 3. Calibration Curve for undialyzed Lysozyme by Bradford Analysis | ||
| Line 36: | Line 37: | ||
* Measure UV-Vis from 400 nm - 800 nm using polystyrene cuvettes. | * Measure UV-Vis from 400 nm - 800 nm using polystyrene cuvettes. | ||
[[Image: Undialyzed_Lysozyme_Calibration.jpg]] | |||
4. Prepare 50 mL 66 mM Potassium Phosphate Buffer | 4. Prepare 50 mL 66 mM Potassium Phosphate Buffer | ||
| Line 51: | Line 53: | ||
* Run Uv-Vis on protein containing solutions that were dialyzed on [http://openwetware.org/wiki/User:Khyra_A._Neal/Notebook/Chem_571/2014/10/08 October 8, 2014] | * Run Uv-Vis on protein containing solutions that were dialyzed on [http://openwetware.org/wiki/User:Khyra_A._Neal/Notebook/Chem_571/2014/10/08 October 8, 2014] | ||
* Run from 200 nm -400 nm | * Run from 200 nm -400 nm | ||
7. Prepare New Dialysis for 30:1 Colloid vs CaCl<sub>2</sub> | |||
* Use 3500 MWCO tubing | |||
* Add 1 mL 30:1 colloid solution to 5 cells on one side of chamber | |||
* Add 1 mL 5 μM, 50 μM, 500 μM, 5 mM, and 50 mM CaCl<sub>2</sub> to the opposite side of the chamber (one concentration per cell) | |||
* Secure wells by screwing them to prevent any evaporation | |||
* Place on low speed shaker overnight | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
Revision as of 20:44, 21 October 2014
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
October 14, 2014Tasklist1. SDS Page #2
3. Calibration Curve for undialyzed Lysozyme by Bradford Analysis
4. Prepare 50 mL 66 mM Potassium Phosphate Buffer
5. Fluorescence on protein containing solutions that were dialyzed on October 8, 2014
6. UV-Vis
7. Prepare New Dialysis for 30:1 Colloid vs CaCl2
| |