User:Khyra A. Neal/Notebook/Chem 571/2014/09/10: Difference between revisions
(fix raw html notebook nav) |
|||
| Line 2: | Line 2: | ||
|- | |- | ||
|style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> Project name</span> | |style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> Project name</span> | ||
|style="background-color: #F2F2F2" align="center"| | |style="background-color: #F2F2F2" align="center"|[[File:Report.png|frameless|link={{#sub:{{FULLPAGENAME}}|0|-11}}]][[{{#sub:{{FULLPAGENAME}}|0|-11}}|Main project page]]<br />{{#if:{{#lnpreventry:{{FULLPAGENAME}}}}|[[File:Resultset_previous.png|frameless|link={{#lnpreventry:{{FULLPAGENAME}}}}]][[{{#lnpreventry:{{FULLPAGENAME}}}}{{!}}Previous entry]] }}{{#if:{{#lnnextentry:{{FULLPAGENAME}}}}|[[{{#lnnextentry:{{FULLPAGENAME}}}}{{!}}Next entry]][[File:Resultset_next.png|frameless|link={{#lnnextentry:{{FULLPAGENAME}}}}]]}} | ||
|- | |- | ||
| colspan="2"| | | colspan="2"| | ||
Latest revision as of 00:18, 27 September 2017
September 10, 2014Bradford AssayPrepare Stock Solutions
UV-Vis AnalysisRecord UV-Vis Spectrum for stock solutions (BSA, Saline Solution, and Tris Buffer)
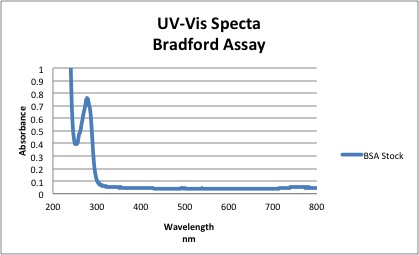
NOTE ALL UV-Vis spectra were run using polystyrene cuvettes. Solutions were discarded in waste bottle and polystyrene cuvettes were placed in a wash tube for cleaning. Data Analysis1. Purity of BSA stock solution Based on the spectra above, the absorbance of BSA at 280 nm = 0.761
|
|||||||||||||||||||||||||