User:Jamie Nunziata/Notebook/Biology 210 at AU
Observing and Identifying Protists in a Hay Infusion Culture
01/28/2015 J.N.
Introduction:
This lab was performed to observe and identify various protists that may be living in our transects. Protists are unicellular eukaryotes that do not perform photosynthesis but instead absorb their own nutrients(Bentley et a., 19). The second group of unicellular eukaryote is algae, which, unlike protists, algae is photosynthetic (Bentley et al. 19). These organisms can also have many different unique characteristics passed on by their particular lineage. These unique characteristics are crucial observations when it comes to identification. One method of organism identification is by using what is called a Dichotomous Key. Dichotomous Keys offer choices on the physical characteristics that may be present in your organisms, such as the organism's shape or color. Those choices may lead you to other choices about your observations, and by the end of it you would have narrowed it down to the species you are observing (Bentley et al. 21). This lab used a Dichotomous Key in order to identify the unicellular eukaryotes we saw in the wet mount slides made from our Hay Infusion culture. To observe any bacteria that may have been present in our culture, agar plates were prepared for observation in our next lab. The purpose of this experiment was to develop techniques for identifying organisms by using a Dichotomous Key and to observe the different protists that may be present in our transect.
Materials and Methods:
To prepare the Hay Infusion culture, our dirt sample collected from our transect was first put into a large jar. 500 mLs of distilled water and 0.1 gram of dried milk was added to the jar with our sample. The mixture was shaken for about 10 seconds and placed into the back of the class, top off, for observation after 1 week of leaving the jar undisturbed. After that week passed, the jar was then collected again and observations were made of the undisturbed environment. Then, the Hay Infusion culture was shaken until it became a homogenous mixture again. Samples were drawn from the culture using a transfer pipette from the top and bottom of the culture and placed on wet mount slides for observation.
In preparation for next lab, we prepared a serial dilution for bacteria plating. To due this, 4 tubes of broth were obtained and labeled 10^-2, 10^-4, 10^-6, or 10^-8. Using a micropippetor, 100 microliters of the hay infusion culture was transferred to the tube labeled 10^-2. The tube was shaken to mix the broth, and 100 microliters was then transferred from the 10^-2 broth to the broth labeled 10^-4 and shaken. 100 microliters was then taken from that broth and transferred to 10^-6, shaken, and then 100 microliters of broth was taken from the 10^-6 tube to the 10^-8. This final tube, 10^-8, is a 1:100000000 dilution. This process is illustrated below:

Agar plates were then made using these tubes of the different dilutions. To prepare these plates, 4 plates of nutrient agar and four plates with the tetracycline added nutrient agar were obtain and labeled, one of each type, 10^-3, 10^-5, 10^-7, and 10^-9. A sample of the broths were spread on the plate by using a glass rod as our inoculating loop. The rod was dipped into ethanol and lit on fire by briefly holding it over a Bunsen burner to sterilize. After the glass cooled, the rod was then dipped into the 10^-2 broth and spread onto the agar plate label 10^-3 for both tetracycline and non-tetracycline. This process was repeated with the rest of the broth, plating the 10^-4 broth onto the 10^-5 plates, the 10^-6 broth onto the 10^-7 plates, and the 10^-8 both onto the 10^-9 plates, making sure to re-sterilize the rod between each plating.
Data:
When first brought back to the station, the Hay Infusion culture had a large amount of dusty sediment on the bottom layer and a large amount of moldy and muddy looking sediment on the top layer. The only apparent sign of life when first looking at the culture was the mold spores growing on the top layer of the jar. There was also a few dead bugs floating at the top and a few dead leaves as well. The culture also smelled of rotten, moldy, and stagnant water. Pictures of the culture can be seen below: 
When observing our wet mount slides, we were able to identify the following organisms from the top and bottom layers of the culture:
Top Niche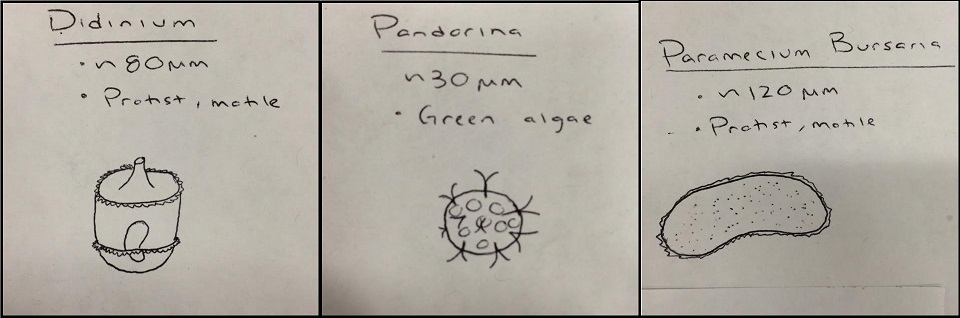
Bottom Niche
Conclusion:
Although there is some overlap, for the most part the organisms living at the bottom of our Hay Infusion culture, as identified with a wet mount slide and a Dichotomous Key, differ from those living on the top layer of our culture. One likely explanation for this could also explain why mud and mold was left floating at the top, and sediment from the dirt and plants lied on the bottom. Our algae species, Pandorina, was found at the top as well. This is because in order for photosynthetic organisms to thrive, such as algae, it needs a light source in order to perform photosynthesis. However, organisms which need to consume their own food, such as the protists identified, would thrive on the bottom of the Hay Infusion where they are able to feed off of any nutrients from the sunken dirt or plant matter. These organisms found in our Hay Infusion would in fact meet all the needs of life according to Freeman. Pandorina, for example, takes in energy though the process of photosynthesis, is capable of replication and transferring the genes of the organism down to other organisms, is comprised of cells, and is capable of evolution.If this Hay Infusion culture were to sit for another two months, one would imagine that most of the organisms left in the culture would be algae species, because eventually the protists will run out of nutrients to consume but the algae can make it's own nutrients with sunlight and CO2 form the open air. However, if the jar was left capped and in the dark, then neither the algae nor the protists would survive an extended period of time.
References:
Bentley, M., Laslo, M., Walters-Conte, K., & Zeller, N. (2015). Aerobic Respiration & Fermentation. In A Laboratory Manual to Accompany General Biology 2 (pp. 18-24). Department of Biology, American University.
Freeman, S. (2010). Biology and the Tree of Life. In Biological science (Fourth ed., pp. 2-4).
Observing a Niche at AU
01/26/2015 J.N.
Introduction:
This lab was performed to serve an introduction to ecology, the branch of biology that studies the different relationships between organisms, species, and the environment (Bentley et al., 2015). In this lab, a transect, or a small section of an ecosystem, was observed to identify any biotic and abiotic factors. Biotic factors are living things, such as moss, and abiotic factors are nonliving things, such as a stop sign. Samples of dirt the various transects were also taken to perform a Hay Infusion Culture that will be observed in the following lab. The purpose of this experiment was to gain a better understanding of the abiotic and biotic factors of different ecosystem by observing specific and unique transects across American University's campus.
Materials and Methods:
The class was first split into groups and assigned different 20 by 20 meter areas of campus to use as their transect. Once each group got their assignment, they went to their assigned area and received a tube from the instructor to take dirt samples from their transect to perform a Hay Infusion Culture in their next lab. The groups then comprised a list of abiotic and biotic factors in their transect along with recording the transect's overall location and topography. Each group member then, independently, sketched their transect to include an overall biological setup of biotic and abiotic factors, along with a having labelled north, south, east and west.
Data:
The transect that was used for this analysis was the Tall Bushes Transect and was located in the middle of an arboretum in between the amphitheater and bender arena. This transect included lamp posts, dirt, and sidewalk cement as its abiotic factors. As far as the biotic factors, this transect contained many different types of ground shrubs and bushes, some small trees, grass, small bugs and microorganisms in the dirt, and some fallen leaves. The overall topography of the transect was that it was flat and higher up in altitude than it's surroundings, and the transect was fairly dry when digging past the top layer of snow. A sketch of the transect can be seen below:

Conclusion:
This lab was aimed toward making observations about a specific ecosystem by analyzing a small sample, or transect, of that ecosystem. The results of this experiment were that most of the biotic factors in our transect were small trees and bushes on the ground, which is an outcome of the fact that our transect was in a dry and high up location. This location was also in an area where their is no immediate water source, also contributing to the observation that the only plants able to sustain life in our transect were ones which could survive in dry areas.
References:
Bentley, M., Laslo, M., Walters-Conte, K., & Zeller, N. (2015). Aerobic Respiration & Fermentation. In A Laboratory Manual to Accompany General Biology 2 (pp. 16-17). Department of Biology, American University.