User:Gabriel H Gan/Notebook/Biology 210 at AU
TA Alyssa Pedersen MW 5:30-8:40PM
Lab 3: Analysis of Bacteria in Transect #3 7/7/14
Introduction
This lab focuses on the study of the bacteria found in transect #3. There are different groups of bacteria, including Proteobacteria, Chlamydiae, Actinobacteria, Spirochetes Cyanobacteria, and Firmicutes (Bentley 2014). Biologists classify bacteria on morphology, and examine characteristics such as form, elevation, margin, size, and stain characteristics (Bentley 2014). A positive gram stain yields bacteria that remains violet after preparation due to the thick peptidoglycan wall, while negative gram stains do not hold the dye (Bentley 2014).
In this lab, the agar plates prepared by serial dilution in lab 2 were analyzed by regular observation with microscopy as well as with gram staining. At the end of lab, PCR was set up for 16s sequencing to be run through gel next lab class.
I do not believe any Archaea species will have grown on the agar plates. Since Archaea tend to grow in extreme environments, the petri dish with agar will be no match. The room temperature petri dish is much cooler than a hot spring, one environment in which Archaea thrive (Bentley 2014).
Materials and Methods
First, all six petri dishes were placed on the bench for visual inspection with the naked eye. From this, it was possible to gather general information about the number of colonies, pattern, colors, and shapes.
Next, wet mounts were prepared from two of the nutrient agar petri dishes and one of the tetracycline petri dishes. These wet mounts were examined under the compound microscope at 1000x using oil.
After, gram stain slides were prepared from the same colonies used for the three regular wet mounts. The colony samples were heat fixed to the slide, stained with crystal violet, and set with Gram's iodine mordant and safranin stain. Observations were recorded under 400x and 1000x.
The final procedure was the PCR setup for 16s sequencing. Bacteria from each of the three colonies used in the wet mount and gram stain was incubated and then centrifuged. Samples from these three tubes were then mixed with a PCR bead and placed into a smaller tube with 16s PCR reaction liquid.
Results
Our group did not check the hay infusion so there is nothing to report on any changes in smell or appearance.
Figure 1: 100 fold Dilutions Petri Dishes (First Four)
These were grown with nutrient agar. Bacteria source is transect 3 hay infusion culture.
Figure 2: 100 fold Dilutions Petri Dishes (Second Four)
These petri dishes contained nutrient agar and tetracycline. Bacteria source is transect 3 hay infusion culture.
Figure 3: 10^-5 Petri Dish
This dish was chosen for microscopy, gram staining, and PCR. The sample was taken from one of the larger yellow colonies.
Figure 4: 10^-9 Petri Dish
This dish was chosen for microscopy, gram staining, and PCR. The sample was taken from the large red colony.
Figure 5: 10^-9 Tetracycline Petri Dish
This dish was chosen for microscopy, gram staining, and PCR. The sample was taken from a larger yellow colony.
Figure 6: View of Gram Stained Sample from 10^-9 Tet Petri Dish
This image shows the gram positive colony of bacteria from the 10^-9 agar and tetracycline petri dish.
Figure 7: View of Gram Stained Sample from 10^-9 Tet Petri Dish
This image shows the gram negative colony of bacteria from the 10^-9 nutrient agar petri dish. The red seen in the photo is from the wax pencil, there is no stained bacteria present.
Figure 8: View of Gram Stained Sample from 10^-5 Petri Dish
This image shows the gram positive colony of bacteria from the 10^-5 nutrient agar petri dish.
Tables and Graphs
Table 1: Results of Serial Dilutions in Nutrient and Nutrient + Antibiotic Agar Plates
This table shows data from colonies counted on plates with different dilutions. Nutrient agar plates had more bacterial colonies observed.
Table 2: Bacteria Characterization of 10^-9 Tet, 10^-9, and 10^-5 Plate Colonies
The data presented above is from the analysis of gram staining the three colonies selected from 10^-9 agar and tetracycline, 10^-9 nutrient agar, and 10^-5 nutrient agar.
Discussion:
The appearance and smell of the hay infusion may change week to week. This is because with time, microorganisms such as protists and bacteria will reproduce. Some are capable of decomposition, and this can cause gases to be released that smell or release byproducts that cloud the water.
There were differences noted between the plates with antibiotic and the plates without antibiotic. All of the tetracycline plates had yellow colonies. In contrast, the nutrient agar plates varied in color, from red to white to yellow colonies. In general, there was more variation noted on the nutrient agar plates, including different edges on the colonies and the presence of a small lawn on the 10^-3 plate. The tetracycline plate colonies appear to have entirely even margins.
The effect of tetracycline on the total number of bacteria is somewhat unclear. Compared to the nutrient agar plates, the 10^-5 and 10^-7 tetracycline plates had no colonies. The 10^-3 and 10^-9 colonies did have more colonies than their sister plates without tetracycline. I believe tetracycline had an effect and limited bacteria growth significantly, however, the reason why the results appear as they are might be due to cross contamination with the spreader when placing the bacteria in the plates. The tetracycline seemed to cause a yellow color change in the bacteria. It appeared that one species of bacteria is unaffected by tetracycline.
Tetracycline antibiotics bind to the 30S ribosomal subunit and prevent t-RNA from binding, which prevents amino acids from being added to the peptide chain, and therefore protein synthesis from occurring (HACCP). Tetracyclines affect both gram-positive and gram-negative species; some microorganisms affected include Rickettsie, Chlamydia, Mycoplasma, spirochetes, and some protozoa, but not fungi or viruses (HACCP). According to the HACCP source, resistance to tetracyclines is widespread.
References
Bentley, M., Walters-Conte, & Zeller, N.K. 2014. A Laboratory Manual to Accompany General Biology II. American University Department of Biology: Washington, D.C.
"Tetracyclines in Veterinary Medicine-Overview". HACCP Manual. n.d. Bio Agri Mix. (8 July 2014). <http://www.bioagrimix.com/haccp/html/tetracyclines.htm>
Lab 2: Identifying Algae and Protists 7/2/14
Introduction
The hay infusion jar from the previous lab period can be considered to be an ecosystem, composed of many microorganisms that each have different niches, or ecological roles (Bentley 2014). To identify organisms, a dichotomous key can be employed. This type of system presents series of two choices that can be used to narrow down the organism in question to a specific species (Bentley 2014). During this lab session, the dichotomous key was employed to determine what types of protists and algae were present in the hay infusion. In addition, a serial dilution was performed to prepare for the next lab session.
Materials and Methods
Before the hay infusion culture was examined, practice using the dichotomous key was obtained by identifying known organisms. After, the hay infusion culture jar was analyzed in terms of odor and appearance. Wet mounts were prepared of samples from both the niche in the top of the jar as well as at the bottom of the jar. Next, the wet mounts were examined under microscope and two organisms from the top of the jar sample and two from the bottom of the jar sample were identified. These organisms were sketched out and pertinent information was recorded. All procedures were completed per the Bio 210 lab manual.
After the hay infusion culture analysis, serial dilutions were performed in preparation for the microbiology lab. A 10^-2, 10^-4, 10^-6, and 10^-8 dilution was made and they were each spread on both a nutrient agar plate and a tetracycline plate. See below diagram of the serial dilution procedure.
Figure 1: Diagram of Serial Dilution Procedure
Serial dilution to inoculate agar petri dishes for bacterial analysis
Results
There was no strong odor coming from the jar with the hay infusion. The smell noted was similar to the odor present around most ponds. The water color observed was cloudy and a mild yellow-green tinge was noted. There was no apparent life on top of the liquid such as mold or green shoots.
In the top of the jar sample, green algae and blepharisma were observed. The sample from the bottom of the jar revealed chilomonas and colpidium. Blepharisma, chilomonas, and colpidium are all protists. The algae observed was green, non-motile, and approx. 50 µm long. The blepharisma was greenish brown with a red brown pigment, non-motile, and approx. 25 µm long. The chilomonas was clear, non-motile, and 37.5 µm long. The colpidium was clear, motile, and 12.5 µm long.
Figure 2: Blepharisma at 400x Magnification
The figure above is a photograph of blepharisma as seen through a compound microscope.
Tables and Graphs
Table 1: Organisms Observed in Hay Infusion of Transect #3 Soil
Table showing results of microscopic examination of transect hay infusion wet mounts.
Discussion: The purpose of this lab was to observe and identify protists and algae from the transect. Samples for wet mounts were taken from the top of the jar and the bottom (two different niches) because they both have different living and nonliving factors. Organisms such as the ones identified compete for space and there is a carrying capacity, or maximum capacity for a species, in a niche (Bentley 2014). It is also possible that some organisms may be found at the top more than others due to the fact that they might need light for photosynthesis. The plant matter can help an organism by providing shelter or food, but it could also be hurtful if it blocks it from needed light.
On page 2 of the Freeman textbook, the needs of life are enumerated. All organisms use energy, are made up of cells, process information, are capable of replication, and evolve (Freeman 2014). Blepharisma, a complex protist discovered in the top of the hay infusion, obtains energy by using its cilia to sweep food into its buccal cavity (Hanna). Blepharisma are unicellular, and store their information in a micronuclei, and can reproduce by binary fission or conjugation (Hanna). Conjugation confers genetic variation through recombination which can permit the organism to evolve (Freeman 2014).
If the hay infusion culture had been observed for another two months, I would predict that more microbes would be visible through microscopy. I also predict that more advanced organisms would appear as the bacteria serve as the food source for other organisms, and it increases over time. There were many selective pressures that affected the community of the samples. First, they were in a space that had a set capacity. In addition, they had to survive with only the nutrients in the water as nothing was added to the jars in the couple of days of incubation. The samples had pressure from the environment, i.e. the lab temperature and light level, to survive, as the temperature and light level in the lab are different from the transect conditions.
References
Bentley, M., Walters-Conte, & Zeller, N.K. 2014. A Laboratory Manual to Accompany General Biology II. American University Department of Biology: Washington, D.C.
Freeman, Scott. 2014. Biological Science. Prentice Hall: New Jersey. 2.
Hanna, Jannette. "Blepharisma". Micscape Magazine. Nov. 2004. Rochester Institute of Technology. (7 July 2014). <http://www.microscopy-uk.org.uk/mag/artnov04macro/jhblepharisma.html>
Lab 1: Observing a Niche at AU 7/1/14
Introduction
The American University campus is saturated with different varieties of ecosystems. This lab notebook entry, the first in a series of three, will present and discuss observations from transect #3. Through this transect, it will be possible to study the biology of communities. The biodiversity, or number and type of species that live in an area, will be analyzed (Bentley 2014). In addition, living (biotic) and nonliving (abiotic) elements will be examined (Bentley 2014). Each species in the community has a niche that is important to the status of the community.
Transect #3 is located between Bender Arena, Hughes Hall, and the Woods-Brown Amphitheater. The coordinates are 38°56'16.2"N 77°05'20.4"W. The transect, approximately 20mx20m, has a collection of bushes, grasses, plants, and trees.
Figure 1: Map of Transect #3 Location on American University Campus
Figure 1: Transect #3 is located in between Bender Arena and Hughes Hall. Map from Google Maps.
Materials and Methods
Visual analysis was completed of transect #3. The transect was walked numerous times and observations on biotic and abiotic factors were recorded. A diagram of the plants and abiotic factors was sketched out. Photos were taken from an aerial view by standing on some of the benches. In addition to the photos taken from above, some were taken at standing height to record the survey of plants, trees and bushes.
Before leaving the transect, a soil and ground vegetation sample was taken. Back in the laboratory, this sample was mixed with Deer Park water and dried milk to make a hay infusion culture per lab manual instructions. The mixture was placed in an open jar and left out to be analyzed in the next lab.
Results
Below is a sketch diagram of the parts of the transect and numerous photographs. The transect is on flat, level ground with the exception of some small beds that are mound shape. In addition to the trees and plants noted, some small flying insects were present in the transect. The trees create a large amount of shady area.
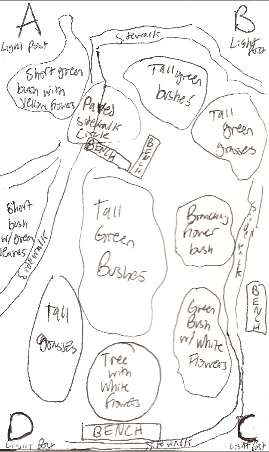
Figure 2: Diagram of Biotic and Abiotic Components of Transect #3
Transect #3 is composed of a sidewalk that loops through an area with grasses, plants, bushes and trees.
Figure 3:
Transect #3 hosts a variety of plant life.
- Note: Aerial photography was difficult due to tall nature of transect and inability to get to a place with a higher vantage point. Instead, multiple images were taken of the variety of elements in the transect.
Tables and Graphs
Table 1: Biotic and Abiotic Components of Transect 3
Discussion:
The purpose of this experiment was to explore the diversity in transcript #3. From initial observations, it appears that there are numerous types of plants and bushes present in the transect. It also appears that there are not many animals living in this transect. This may be due to the fact that the area is right next to the road and a busy part of campus that may not be conducive to undisturbed living.
The results of this initial exploration will be used to further the study of the many levels of organisms in the transect. By studying a small area with biodiversity, one can learn more about ecology.
References
Bentley, M., Walters-Conte, & Zeller, N.K. 2014. A Laboratory Manual to Accompany General Biology II. American University Department of Biology: Washington, D.C.