User:Claudia J. Woloshchuk/Notebook/Biology 210 at AU
Thursday, January 29, 2015: Lab 3 Microbiology and Identifying Bacteria with DNA Sequences
Purpose: The purpose of this lab was to understand the characteristics of bacteria, to observe resistance and to understand how DNA sequences are used to identify species. The hypothesis is that bacteria will grow on the all of the agar plates, including the ones with tetracycline due to bacterial resistance. In addition, Archaea species will have grown on the agar plates because they tend to grow in extreme, isolated environments and the agar plates provide that.
Materials and Methods: In order to understand bacteria and observe resistance bacteria from the Hay Infusions were placed on 8 serial dilutions. Four of the serial dilutions had just an agar nutrient and the other four had agar nutrient and tetracycline. Therefore if any bacteria grew on the agar plates with tetracycline, it would be understood that that bacteria was antibiotic resistant. These plates were prepared one week before this lab was completed. The bacteria that had grown was observed and recorded, taking into account the dilution, agar type, colonies counted, and the colonies per mL. Bacteria cell morphology was the observed using the bacteria from the agar plates, including the plates with tetracycline if bacteria had grown. This was done by creating four wet mounts using two bacterial samples from the agar plates without tetracycline and two samples from the agar plates with tetracycline. To crete a wet mount one sterilizes a loop over a flame, scrapes a tiny amount of growth from the surface of the agar plate and mix it with a drop of water on a slide. Then place a cover slip over the slide. The bacteria was observed under a microscope at 10x and 40x. Then oil was used to observe the slides at 100x. Four gram statins were then made using bacteria samples from the same colonies within the agar plates. In order to prepare these slides one first sterilizes a loop over a flame, scrapes up a tiny amount of growth from the agar plate and mixes it with a drop of water on a slide. The pass the slide, bacterial smear side up through a flame three times. Next, working over a staining tray, cover the bacterial smear with crystal violet for 1 minute. Rinse the stain off with a bottle of water. Cover the bacterial smear with Gram's iodine mordant for 1 minute. Rinse the stain off using a bottle of water. Next, decolorize by flooding the bacterial smear with 95% alcohol for 10-20 seconds. Rinse gently with water. Then cover the smear with safranin stain for 20-30 seconds. Rinse the stain off with water. Blot the excess water carefully using a kimwipe. Coverslips were not used. This process was completed four times to make four slides. The slides were then observed using a microscope with 10x and 40x. Lastly, PCR set up was completed for 16s sequencing. In order to do this first transfer a single colony of bacteria to 100 μL of water in a sterile tube. THen incubate at 100 degrees celcius for 10 minutes in a heating block. Next centrifuge the samples for 5 minutes at 13,400 rpm. During the centrifugation, add 20 μL of primer/water mixture to a labeled PCR tube. Mix it to dissolve the bead. Lastly transfer 5 μL of supernatant from the centrifuged samples to the 16s PCR reaction and place the tube in the PCR machine. These was done 4 times with 4 different bacterial samples from the agar plates.
Data and Observation: One last observation of the Hay Infusion Culture was made. There was only 1 inch of water left which was filled with soil, berries, and moss. It still smelled rotten. The appearance and smell of the infusion may change week to week due to the growth and death of organisms within the infusion and due to the evaporation of the water.
Table 1: 100-fold Serial Dilutions Results Dilution Agar type Colonies Counted Conversion Factor Colonies/mL 10^-3 nutrient 64 x10^3 64,000 10^-5 nutrient 22 x10^5 2,200,000 10^-7 nutrient none x10^7 0 10^-9 nutrient none x10^9 0 10^-3 nutrient + tet 71 x10^3 71,000 10^-5 nutrient +tet 1 x10^5 100,000 10^-7 nutrient+tet none x10^7 0 10^-9 nutrient +tet none x10^9 0
Image 1: Agar plate 10^-3 without Tet

Image 2: Agar plate 10^-5 without Tet

Image 3: Agar plate 10^-3 with Tet

Image 4: Agar plate 10^-5 with Tet

Table 2: Bacteria Characterization Colony Label Plate type Colony Description Cell Description Gram + or - Additional Notes
10^-5 Nutrient Milky white, circular N/A - Large clumps/circular
Convex, smooth magnification=40x
10^-5 Nutrient Yellow, wavy edges, N/A - rods clumped together
convex, smooth magnification=40x
10^-3 Nutrient + Tet Yellow, round, convex Round shape + circular clumps
Smooth slow motility, magnification=40x
Clumped together
10^-3 Nutrient + Tet Milky white, circular Thread like, single - rods clumped together
Convex, smooth Moves fast, randomly magnification=40x
Conclusion:
In conclusion the plates with tetracycline grew bacterial growth including Archaea growth as hypothesized. The data as presented above supports this conclusion. The growths looked to be the same type of growths as on the plates without tetracycline (Images 1-4). Although the 10^-5 agar plate with Tetracycline had far fewer growths than the 10^-5 agar plate without Tet, the 10^-3 agar plate with Tet had more growths than the 10^-3 agar plate without Tet. This indicated that the bacteria that did grow was antibiotic resistant.The serial dilutions for 10^-7 and 10^-9 did not grow any growths (table 1). Tetracycline is a broad spectrum antibiotic which works by inhibiting cell growth by inhibiting translation. Tetracycline will bind to the 16S part of the 30S ribosomal subunit and prevent the amino-acyl tRNA from binding to the A site of the ribosome. Some bacteria that are sensitive to Tetracycline are gram-positive bacteria, gram-negative bacteria, chlamydiae, mycoplasmas, rickettsiae, and protozoan parasites, although more micro organisms are becoming resistant as shown in this experiment (http://www.ncbi.nlm.nih.gov/pmc/articles/PMC99026/). One thing that may have caused experimental error is human error. This is in relation to the number of colonies on the agar plates.
-C.W.
1.27.15
Good first entry. For future entries try to organize lab book into: Purpose, Materials & methods, data & observations and conclusion future directions. You also need to address the red text from the manual.
SK
Thursday, January 22, 2015: Lab 2 Hay Infusion Culture
The Hay Infusion Culture, filled with soil and ground vegetation from the transect, smelled rotten (similar to a dirty fish tank). At the top of the jar there is floating berries, a wood chip and a layer of what looks like slime. This layer could be algae. At the bottom of the jar there is soil, moss, and some pieces of wood. In the middle of the jar there is dirty water with small bits of brown debris floating in it.
We took two samples for microscopic observation. One came from the very top layer of the jar with the layer of algae and the other came from near the moss at the bottom of the jar. The organisms may differ based on the location that we pulled samples form due to the fact that some organisms may prefer an area with more light and algae and others may prefer an area with more soil and plant matter.
From the wet mount made from the sample taken from the bottom of the jar we observed a Colpidium. This organism was motile, is a protozoa, non-photosynthesizing and consumes nutrients. It measured to be 26 μM. We found many of these organisms, around 20, on the slide that was made from the sample taken from the top of the jar as well.

From the wet mount made from the sample taken from the top of the jar we observed Gonium. This protozoa contained about 16 cells and was 20μM. Gonium are motile and are non photosynthesizing.

The Gonium that we found meets all the needs of life because it is alive. It is a live due to the fact that it acquires and uses energy, is made up of one or more cells (ours was made of 16 cells), it processes information, is capable of oogamous replication, and it is a product of evolution because it is part of the volvocine line.
If the Hay culture grew for another two months I predict that different organisms would develop and the organisms that were found already would grow, multiply and some would die.
One selective pressure that would affect the community of our samples would be the amount of sunlight let into the Infusion Jar, which would effect the photosynthesis of some organisms in the culture. Another selective pressure would be the variety of organisms living in the culture. A community needs a variety of organisms to go on and if organisms die off or were never there, this may be detrimental to the community.
-C.W.
Thursday, January 15, 2015: Lab 1 AU Transect
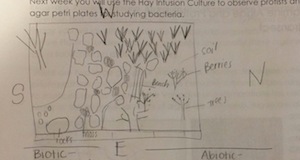
My lab group, Group 2, was assigned a 20 by 20 meter transect on American University's campus. The transect is located in between two sidewalks and a dorm building. It is part of a garden with benches, grass, many plants and trees.
Biotic components: trees, moss, bushes, shrubs, grass, berries Abiotic components: stepping stones, arboretum signs, shade from buildings, benches, pebbles, soil, the little bit of snow
-C.W.
Thursday, January 15, 2015: Observing Evolution
| Organism | Size | Reproduction | |
| chlamy | 10 | iso | |
| gonium | 50 | oo | |
| volvox | 214 | oo |
-C.W.