User:Christopher D. Klocke/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| Line 83: | Line 83: | ||
Conclusions: | Conclusions: | ||
From our observations, we can infer that paramecium aurelia prefer to reside in a niche that is farther underwater, near plant material. We can also infer that calpidium and pandorina are comfortable in a variety of niches within the hay infusion culture, as they were found in two separate niches. I would assume that if the hay infusion were allowed to develop for another two months, the microorganisms would be found to reside in more segregated, specific niches instead of mixed around as some of them were. | From our observations, we can infer that paramecium aurelia prefer to reside in a niche that is farther underwater, near plant material. We can also infer that calpidium and pandorina are comfortable in a variety of niches within the hay infusion culture, as they were found in two separate niches. I would assume that if the hay infusion were allowed to develop for another two months, the microorganisms would be found to reside in more segregated, specific niches instead of mixed around as some of them were. | ||
Lab 3 - Microbiology and Identifying Bacteria with DNA | |||
Questions: | |||
Will any Archaea have grown on the agar plates? Why will hay infusion culture appearance or smell change over the course of a week? What bacteria can be observed in the cultures, how are they affected by the presence of antibiotic, and how do they respond to gram staining? | |||
Procedure: | |||
We observed our hay infusion culture and noted any further observable changes. | |||
We then observed the agar plates on which we plated samples from our hay infusion culture. Since they have been allowed to grow for a week, we will expect to see some bacteria growth on some of the plates. We noted whether the presence of the antibiotic tetracyclin had any effect on the growth of bacterial colonies on the agar plates. | |||
We then used a loop to take a small bacteria sample from each agar plate and prepare wet mount slides for microscopy. We observed the samples and noted their observable characteristics. | |||
We then took a second bacterial sample from each plate and gram-stained them. By putting them through a series of dyes, we exposed the cells to safranin stain, which will stain the membrane of gram-negative cells, and crystal violet stain, which will stain the peptitoglycan of gram-positive cells. We observed the prepared slides under microscopes in order to determine whether they were gram-positive or gram-negative. | |||
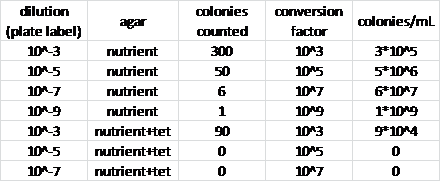
Observations/Results: | |||
[[Image:Biolab_-_bacteria_data_table.png]] | |||
Conclusions: | |||
We can infer that the bacteria that were able to grow on one of the tetracyclin-infused-agar plates were antibiotic resistant. We can also infer certain properties about the bacterial colonies due to the gram staining: the cells that seemed to be colored with the red safranin dye most likely had two membranes and a thin peptidoglycan wall, while the cells that appeared purple from the crystal violet stain probably had a thicker peptidoglycan cell wall and no second membrane on the outside. | |||
Revision as of 13:33, 16 February 2014
Part 1: The Volvocine Line
Questions:
What differences can be observed between the various types of organisms in the Volvocine line? What are some possible explanations for these observed differences?
Procedure:
1)My lab group obtained a clean slide 2)we used a transfer pipette to add one drop of water containing Chlamydomonas to the slide 3)we added a drop of Protoslow solution in order to make the organisms easier to observe 4)we placed a cover slip over the drops and placed the slide under the lens of a microscope 5)we observed the organism - after viewing the organisms at several lens objectives, we were able to see individual colonies of cells 6)we repeated this procedure for the Gonium and Volvox subjects.
Observations/Results:
When my lab group observed the Chlamydomonas population, we saw single-celled organisms that were grouped into colonies of about 25.
When we observed the Gonium population, we saw organisms composed of about six cells each, in colonies of 6 or so organisms.
When we observed the Volvox population, we saw organisms made up of around 150 cells each, in colonies of one to three organisms.
Conclusions:
The organisms of the Volvocine line that were observed could be seen as getting progressively more complex; this exhibits the way in which related organisms can descend from a common ancestor and adapt in order to fill a particular ecological niche. The more complex organisms in the volvocine line are observed as having different types of cells serving specialized functions within each individual organism.
Part 2: The AU Transect
Questions:
What biotic/abiotic components can be found within your given transect, and how do they interact with one another?
Procedure:
My lab group walked out to our transect, which takes form as a 20'x20' square of land on American University's campus. We visually studied the area from a variety of angles and took a sample of soil and vegetation for further study in the future.
Observations/Results:
Our transect can be found between American University's main road, Hughes Hall, and the Amphitheater. It is located in the middle of the garden, between two of the winding cement paths. This area is shaded by small trees and bushes, and many organisms can be observed. The terrain is fairly flat, and the ground is covered by soil, mulch, and low plants in the places where it is not paved.
As far as biotic components of our transect, my group observed a bird, several squirrels, a low, green, leafy ground cover plant, patches of tall, tan grass, and several large shrubs. When looking for abiotic components, we observed two metal light poles, some loose trash (chip bags, etc.), a cement sidewalk, soil, and mulch.
Conclusions:
We observed a number of biotic and abiotic components in our transect. Today, we focused on the larger, visible components, but we collected a sample in order to observe the microscopic elements of our transect in the future. Upon further observation, we should be able to see what microscopic organisms inhabit this part of American University's campus.
Very good Lab 1 entry. Nice description of tasks and conclusions. SK
Lab 2: Identifying Algae and Protists
Questions: Can protists be identified under microscopy by noting their size, movement, shape and color? What microorganisms can be found in various niches of a hay infusion culture produced from our transect last week? Are certain organisms specific to certain niches, or are some of them found everywhere?
Procedure:
Part 1) Identifying Protists: For this stage my group studied and identified three different protists. We measured them under a microscope and took note of their color and the speed they moved. Using a chart of protists, we attempted to identify each organism.
Part 2) Studying Hay Infusion Cultures: We used basic sight and smell to take general observations of our hay infusion culture, then we used pipettes to take water samples from several niches in the ecosystem and observed their microorganism content with microscopy.
Part 3) Serial Dilutions and Agar Plating We also performed a set of serial dilutions in order to prepare agar plates for the organisms to grow on in various concentrations. We plated these dilutions onto agar plates with and without the antibiotic tetracyclin. These were set aside for future study, once they have developed.
Observations/Results:
Part I: The first organism we studied was a medium-speed, three hundred micron long green protist that we determined to be a paramecium. The second organism was a fast, thirty micron long protist that appeared to be a pandorina. The third was a medium-speed, green, ovular protist, about seventy microns long, that we determined to be a euglena.
Part II: At first look, we noticed a rotting smell coming from our hay infusion culture and a thin, elastic film across the surface of the liquid. We then took samples from two different niches, near the plants at the bottom and just beneath the film at the surface. We found three types of organisms by the film at the top. One of them was a seventy micron, medium speed protist. Another was a forty micron, fast moving protist that we determined to be a pandorina. The third was an eighty micron, immobile or very slow organism that we determined to be a calpidium.
In the second niche, near the plant material toward the bottom, we found a 100 micron, gree, slow calpidium, a light green, eighty micron, fast-moving pandorina, and a very fast, light green, 180 micron long paramecium aurelia.
Conclusions: From our observations, we can infer that paramecium aurelia prefer to reside in a niche that is farther underwater, near plant material. We can also infer that calpidium and pandorina are comfortable in a variety of niches within the hay infusion culture, as they were found in two separate niches. I would assume that if the hay infusion were allowed to develop for another two months, the microorganisms would be found to reside in more segregated, specific niches instead of mixed around as some of them were.
Lab 3 - Microbiology and Identifying Bacteria with DNA
Questions:
Will any Archaea have grown on the agar plates? Why will hay infusion culture appearance or smell change over the course of a week? What bacteria can be observed in the cultures, how are they affected by the presence of antibiotic, and how do they respond to gram staining?
Procedure:
We observed our hay infusion culture and noted any further observable changes.
We then observed the agar plates on which we plated samples from our hay infusion culture. Since they have been allowed to grow for a week, we will expect to see some bacteria growth on some of the plates. We noted whether the presence of the antibiotic tetracyclin had any effect on the growth of bacterial colonies on the agar plates.
We then used a loop to take a small bacteria sample from each agar plate and prepare wet mount slides for microscopy. We observed the samples and noted their observable characteristics.
We then took a second bacterial sample from each plate and gram-stained them. By putting them through a series of dyes, we exposed the cells to safranin stain, which will stain the membrane of gram-negative cells, and crystal violet stain, which will stain the peptitoglycan of gram-positive cells. We observed the prepared slides under microscopes in order to determine whether they were gram-positive or gram-negative.
Observations/Results:
Conclusions:
We can infer that the bacteria that were able to grow on one of the tetracyclin-infused-agar plates were antibiotic resistant. We can also infer certain properties about the bacterial colonies due to the gram staining: the cells that seemed to be colored with the red safranin dye most likely had two membranes and a thin peptidoglycan wall, while the cells that appeared purple from the crystal violet stain probably had a thicker peptidoglycan cell wall and no second membrane on the outside.