User:Asya L. Tucker/Notebook/Asya 571/2015/11/04: Difference between revisions
From OpenWetWare
(Autocreate 2015/11/04 Entry for User:Asya_L._Tucker/Notebook/Asya_571) |
No edit summary |
||
| Line 6: | Line 6: | ||
| colspan="2"| | | colspan="2"| | ||
<!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | ||
== | ==Objective== | ||
#Measure protease Trypsin's kinetics with the fluorescence assay using 1nM of trypsin at different heating intervals. | |||
#Create a calibration curve using fluorescence for trypsin. | |||
==Procedure== | |||
The general protocol detailed in Dr. Hartings' [[User:Matt_Hartings/Notebook/AU_Biomaterials_Design_Lab/2015/10/06|lab notebook]] was used. The following specific steps were performed: | |||
*Protease Sample Prep. | |||
#Used eppendorf tube no. 6 that weighed (1.01922 )g, and contained (0.00128)g of trypsin. | |||
#Added 1mL of phosphate buffer. | |||
#Final concentration: (54.93 µM | |||
#We diluted the 54.93 uM )Trypsin sample by pipetted 0.0182 mL to 0.982 ml of phosphate buffer to make 1 uM solution of Trypsin. | |||
#We pipetted 0.01 mL to make 0.01 uM solution of Trypsin. | |||
*Sample Prep. | |||
#Used 7 eppendorf tubes, each containing gold fibers. | |||
#To each tube add: | |||
##0.9981mL of buffer | |||
##0.0019mL of trypsin (add this at the time of putting the tubes in the 37˚C hot water bath). | |||
*Blank Prep. | |||
#In on eppendorf tube add: | |||
##0.9981mL of phosphate buffer | |||
##0.0019mL of trypsin solution | |||
*Additional specifications: | |||
#Prior to prepping the samples, the eppendorf tubes containing the fibers were centrifuged for 10 mins at 300rpm. | |||
#After heating the samples, they were all centrifuged for 1 min. at 13'200rpm. This was done only for the samples, not the blanks. | |||
Calculations: | |||
V1 = [(0.1µM)(1mL)]/52.36µM = 0.0019mL, amount of trypsin solution needed | |||
Volume of buffer: 1mL - 0.0019mL = 0.9981mL | |||
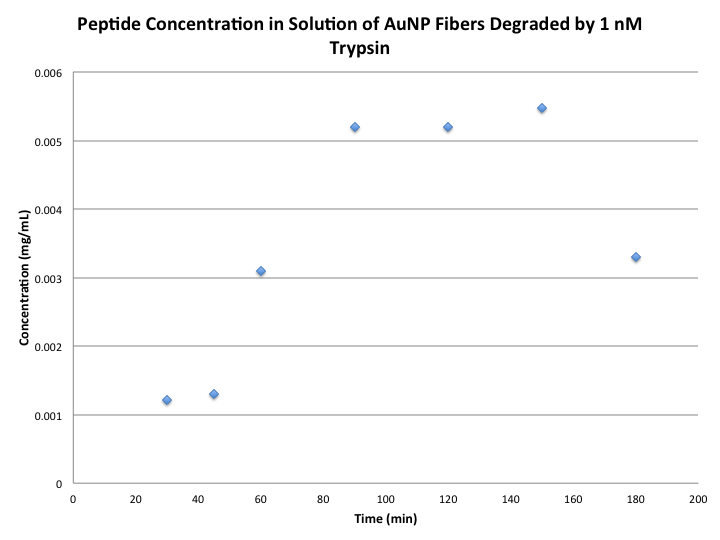
==Data== | |||
[[Image:RAM_fluorescence1nmnov4.png]] | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
Revision as of 06:46, 1 December 2015
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
Objective
ProcedureThe general protocol detailed in Dr. Hartings' lab notebook was used. The following specific steps were performed:
Calculations: V1 = [(0.1µM)(1mL)]/52.36µM = 0.0019mL, amount of trypsin solution needed Volume of buffer: 1mL - 0.0019mL = 0.9981mL Data | |