User:Ariana Leonelli/Notebook/Biology 210 at AU: Difference between revisions
No edit summary |
No edit summary |
||
| (36 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
'''March 22''' : Embryology and Zebrafish Development | |||
The objectives of this lab were to learn the stages of embryonic development, compare embryonic development in different organisms, and set up an experiment to study how environmental conditions affect embryonic development. Three procedures, each focusing on observing a different animals development (starfish, frog and chick) are going to aid in accomplishing the three objectives above. This lab will also feature the results of the Zebrafish experiment my group did focusing on salinity. | |||
Table 1 Comparison of Embryological Features of a Developing Sea Star, Frog, and Chick | |||
*no ocular micrometer for sea star | |||
[[Image:Screen_Shot_2014-03-22_at_1.19.57_PM.png]] | |||
Table 2 Comparison of Ecological Aspects in Starfish, Frogs, and Chick | |||
[[Image:Screen_Shot_2014-03-22_at_1.28.56_PM.png]] | |||
'''Zebrafish Experiment''' : This was carried out over a two week period where we tested the effects of salinity on developing embryos of zebrafish. There was definitely a more noticeable difference in the effects of the salinity on the ebryos the higher the concentration of salt there was in the petri dish. Zebrafish were chosen because of their ability to reproduce and how easy it is to keep track of what you are doing to them. Also their embryos are transparent, making it easier to see what changes are happening in the embryo while at the same time being able to absorb whatever is in the water they are developing in very easily. It should also be noted that on Day three of observation we needed to make new concentrations due to the fact that all of the zebrafish exposed to the 3% salinity for the first two days had died only a few hours after we put them in a petri dish. The new concentrations we made were 3% salinity, 0.3% salinity, and 0.03% salinity. Below is the raw data from the experiment we conducted. | |||
Methods: | |||
Day 1: On day one we set up our experiment by randomly selecting 40 embryos and evenly placing them in two different petri dishes. One had deerpark water in it while the other had a concentration of 3% NaCl. Once set up and the embryos were staged we were able to finish for the day after making our observations. | |||
Day 3: We needed to make new concentrations due to the fact that all of the zebrafish exposed to the 3% salinity for the first two days had died only a few hours after we put them in a petri dish. The new concentrations we made were 3% salinity, 0.3% salinity, and 0.03% salinity. We restaged all of the fish and then made observations again that are on the table below. | |||
Day 5: We removed the dead fish embryos that we found and then added more water to the control and more concentrated saline solution to their respective petri dish. We staged the fish once more and then made our observations that are in the chart below. | |||
Day 7: Once again we needed to remove the dead fish embryos and then replace the water as we did before. On day seven there were no longer any fish left with the 3% salinity concentration dish. The observations made on that day are in the results table below. | |||
Day 9: Today was the first day that we started fixing dead fish embryos. We fixed the same amount of fish from each plate whether dead or alive so we could compare them later on in our study with one another. The water was replaced again with the appropriate concentration with the observation recorded below. | |||
Day 11: At this point all of the 0.3% NaCl fish embryos had died so we fixed more fish and then proceeded to refill the petri dishes with their correct concentrations of NaCl. The staging results and observations are below. | |||
Day 14: This was the last day of observation where we fixed the rest of our fish embryos and then cleaned up all of the materials used. We then were able to observe our fixed embryos which is also recorded below. | |||
Results: | |||
[[Image:Screen_Shot_2014-03-22_at_1.36.31_PM.png]] | |||
[[Image:Screen_Shot 2014-03-22_at_1.39.28_PM.png]] | |||
[[Image:Screen_Shot_2014-03-22_at_1.44.06 PM.png]] | |||
The below table shows the observations on our fixed slides of the zebrafish embryos | |||
[[Image:Screen Shot 2014-03-22 at 2.00.56 PM.png]] | |||
Discussion: | |||
The most important observations and results were probably on day three and day eleven / fourteen. Day three was significant because this was when we needed to reset our entire experiment since our testing group had all died and add some variables so that we could actually make some observations to determine what happens when an embryo is developing in that environment. Day eleven / fourteen was interesting as well since a lot of the control died off suddenly but we attribute this to a lack of nutrients since the majority of the class also experienced this same drop off. We also noticed that the embryos in control started to have greenish black eye pigmentation where the other embryos in testing group only had black pigment in their eyes. Obviously too much salt is not good for anybody but the amount of salt a human would have to consume to create a saline environment would be very high. I would say this study has more of an implication of fish who are developing in the incorrect environment than on actual people. Limitations would be the amount of time and uncontrollable weather conditions throughout the experiment but in terms of improvements or suggestions I think it would be interesting to see if these fish embryos can reproduce normally and lead a normal life even though the environments they developed in arent their natural ones. | |||
ATL | |||
'''February 26''' : Mini Lab Entry (Mini Lab oriinally on February 26) | |||
The objective of this lab was to analyze the results of the PCR that we did a couple weeks ago to deermine what bacteria is most prominent in our transects. Sample two in the chart below is my groups sample but the unknown sample is from the same transect but a different group because the first sample we tried to use PCR on did not work. | |||
[[Image:Screen_Shot_2014-02-28_at_2.12.50_PM.png]] | |||
Table 1: Table of Results for PCR | |||
ATL | |||
'''February 26''' : Fifth Lab Entry - Invertebrates (Lab 5 originally on February 12) | |||
The main objectives of this lab are to uderstand the importance of invertebrates and to learn how sumple systems (including specialized cells and overall body plan) evolved into more complex systems. The following three procedures help accomplish these objectives. | |||
Procedure 1: Observing Acoelmates, Pseudocoelmates, and Coelmates - In this procedure we were required to observe and describe the movements of the three types of worms and how the movement relates to their body structure. The first structure I observed was the nematode ascaris cross section which had no movement and is shown below (Figure 1). The second structure I observed was the nematode cephalobus whcih was the live section of the worm that had some movement (Figure 2). The last figure that we looked at for the nematode in this procedure was the coelmate cross section (Figure 3). | |||
[[Image:Screen_Shot_2014-02-26_at_6.03.38_PM.png]] | |||
Figure 1 | |||
[[Image:Screen_Shot_2014-02-26_at_6.04.09_PM.png]] | |||
Figure 2 | |||
[[Image:Screen_Shot_2014-02-26_at_6.07.57_PM.png]] | |||
Figure 3 | |||
Next for this procedure we needed to observe planaria (flat worms) and do the same thing as we did for the earthworms above. The first image of planaria observed was an acoelmate cross section (Figure 4). Following that, I observed a planaria that was stained so i could see the digestive tract under the microscope (Figure 5). Lastly I observed the live section of the planaria and I noticed that it slides along the petri dish in order to move around (Figure 6). | |||
[[Image:Screen_Shot_2014-02-26_at_6.08.05_PM.png]] | |||
Figure 4 | |||
[[Image:Screen_Shot_2014-02-26_at_6.08.15_PM.png]] | |||
Figure 5 | |||
[[Image:Screen_Shot_2014-02-26_at_6.08.24_PM.png]] | |||
Figure 6 | |||
Procedure 2: Analyzing the Invertebrates Collected in the Burlese Funnel - In this procedure we needed to observe any invertebrates that we found after using the Burlese funnel on our Hay Infusions. Unfortunately due to the weather leading up to when our samples were collected, all of the invertebrates that were in our transect were mostly washed away because of the location of the transect and the angle it is at. Due to this we needed to observe the west virginia samples that were provided incase something like this were to happen. We selected five of them and the following is what we observed. | |||
Organism 1: Flea - 2 antennae, 6 legs, 2 body sections, ~3 mm | |||
Organism 2: Fly - house fly, 2 wings, 2 body segments, ~5 mm | |||
Organism 3: Fly - fruit fly, 2 wings, one body segment, ~2 mm | |||
Organism 4: Termite - soft body, white, no wings, ~1 cm | |||
Organism 5: Biting lice - 4 legs, no antennae, 2 body segments, no wings, ~2 mm | |||
Procedure 3: Vertebrates and Niches - In this part we were asked to think of possible vertebrates that we might find in or around our transect. We were also asked to determine the classification of these along with identifying biotic and abiotic factors that would benefit each species mentioned. Finally we needed to make a food web of the organisms that we mentioned may appear at our transect. Five organisms that may be at my transect, the mini marsh, are the song sparrow, robin, chipmunk, raccoon, and the easter grey squirrel. Below is information on each of the organisms that may be found in my transect. | |||
Song Sparrow: | |||
-Phylum: Chordata | |||
-Class: Aves | |||
-Species: M. Melodia | |||
-Genus: Melospiza | |||
-Biotic: worms in soil are food | |||
-Abiotic: water in transect, dead twigs and leaves for nests | |||
Robin: | |||
-Phylum: Chordata | |||
-Class: Aves | |||
-Species: T. Migratorious | |||
-Genus: Turdus | |||
-Biotic: worms in soil are food | |||
-Abiotic: water in transect, dead twigs and leaves for nests | |||
Chipmunk: | |||
-Phylum: Chordata | |||
-Class: Mammalia | |||
-Species: T. Striatus | |||
-Genus:Tamias | |||
-Biotic: bulbs from plants in transect for food | |||
-Abiotic: water in transect | |||
Racoon: | |||
-Phylum: Chordata | |||
-Class: Mammilia | |||
-Species: P. Lotor | |||
-Genus: Procyon | |||
-Biotic: the invertebrates for food that are in the transect | |||
-Abiotic: water in transect | |||
Eastern Grey Squirrell: | |||
-Phylum: Chordata | |||
-Class: Mammalia | |||
-Species: S. Carolinensis | |||
-Genus: Sciurus | |||
-Biotic: seeds from transect as food | |||
-Abiotic: water in transect, bark in transect for food | |||
Food Web With Animals Relevant to Our Transect: | |||
Robin and Song Sparrow --> Earthworms --> Bacteria and archaea --> Dead leaves | |||
ATL | |||
'''February 26''' : Fourth Lab Entry - Plantae and Fungi (Lab 4 originally on Fecruary 5) | |||
The main objectives of this lab were to undterstand the characteristics and diversity of plants, and to appreciate th function and importance of fungi. These objectives were accopmplished by the following five procedures that are discussed below. | |||
Procedure 1 + 2: Collecting Five Plant Samples From the Transect, and Plant Vascularization - We needed to go over to our transect in front of Kogod School of Business and collect five different plant samples from the transect. Below is a table describing the different vegetation from our transect and all of the information about them that we were required to find. Also below is a picture of the transect so you can have a better understanding of where each of the plants were taken from. Procedure 2 asks for a description of the vascularization of each plant we found which is also in the table below. | |||
[[Image:Screen_Shot_2014-02-26_at_4.24.04_PM.png]] | |||
Table 1: This is a table of the five plants we selected and the information about them and where they were found. | |||
[[Image:Screen_Shot_2014-02-26_at_4.30.06_PM.png]] | |||
FIgure 1: This is a picture of the transect for a reference as to where we got our plants. | |||
Procedure 3: Plant Specialization - In this procedure it was necessry for us to describe the shape, size and cluster arrangement of the leaves from each of the transect plants. | |||
Plant #1: There were no leaves on the cat tail and based on the surrounding environment no evidence that there were leaves on the plant. | |||
Plant #2: The leaves on the tall light brown grass type plant were very small and narrow. They were also very numerous and were only on the ends of each stem. | |||
Plant #3: The third plant had broad leaves which were dark in color with visible veins. This was the red bush. | |||
Plant #4: The fourth plant had round green leaves in which the veins were visible. This was the green ground plant. | |||
Plant #5: The fifth plant was grass so it only had one leaf (the blade of grass) which was straight and narrow with no veins visible. | |||
Procedure 4: Plant Reproduction - Next we needed to identify each of the plants we found in our transect as monocot or dicot. After observing each of the plants and their seeds or their buds if the had them, we were able to determine which of the two each of our plants were. All of the plants we looked at except for the grass, were dicot. The grass however, was monochot. We also observed the lily flowers provided in class and we determined those to be monocots. | |||
Procedure 5: Observing Fungi - In this procedure we observed a fungi presented to us on a petri dish that we needed to observe. The fungi that we observed happened to be bread mold. We needed to determine the importance of fungi sporangia as well. I knew this was a fungus that I was looking at because I could see the mycellum and sporangia under the desiction scope and these are two things that you often find in fungi. The sporangia is important to fungi because it forms spores for the fungi, which is used for asexual reproduction in the fungi. Below is a picture of the bread mold. The black dots are the sporangia. | |||
[[Image:Screen_Shot_2014-02-26_at_5.09.35_PM.png]] | |||
Figure 2: Bread mold | |||
ATL | |||
'''February 16''' : Third Lab Entry - Microbiology and Identifying Bacteria with DNA (Lab 3 originally on January 29) | '''February 16''' : Third Lab Entry - Microbiology and Identifying Bacteria with DNA (Lab 3 originally on January 29) | ||
The main objectives of this lab were to understand the characteristics of bacteria, to observe antibiotic resistance, and to understand how DNA sequences are used to identify species. The three objectives that I have stated were accomplished by the following three procedures described below. | The main objectives of this lab were to understand the characteristics of bacteria, to observe antibiotic resistance, and to understand how DNA sequences are used to identify species. The three objectives that I have stated were accomplished by the following three procedures described below. | ||
Procedure 1: Quantifying and Observing Microorganisms - We needed to first make another set of observations from our transects that we had made on the first lab of the semester. After we had done that, we then needed to observe the petri dishes that we had prepared the previous class from the serial dilutions of the Hay Culture. We needed to observe all seven dilution plates, four of which with a nutrient agar, and three of which with a nutrient and tetracycline (antibiotic) agar. For each petri dish we had to record the number of colonies in each dish and record them in a table. | Procedure 1: Quantifying and Observing Microorganisms - We needed to first make another set of observations from our transects that we had made on the first lab of the semester. After we had done that, we then needed to observe the petri dishes that we had prepared the previous class from the serial dilutions of the Hay Culture. We needed to observe all seven dilution plates, four of which with a nutrient agar, and three of which with a nutrient and tetracycline (antibiotic) agar. For each petri dish we had to record the number of colonies in each dish and record them in a table. I do not think that we would find any archaea on the petri dishes since they only grow in harsh environments and our transects were not considered harsh environments. The appearance and smell of the transect may have changed week to week because new bacteria may be developing within, and things may be decomposing etc. | ||
Hay Culture Observations: | Hay Culture Observations: | ||
| Line 23: | Line 238: | ||
Procedure 2: Antibiotic Resistance - In this procedure we were required to observe our petri dishes once again along with our recorded table in order to make comments about the appearances and differences in between the plates with nutrient agar and the plates with nutrient and antibiotic agar. After observing all of the collected information, my group noticed the following: | Procedure 2: Antibiotic Resistance - In this procedure we were required to observe our petri dishes once again along with our recorded table in order to make comments about the appearances and differences in between the plates with nutrient agar and the plates with nutrient and antibiotic agar. After observing all of the collected information, my group noticed the following: | ||
Plates with Nutrient Only: no fungi, more colonies, smaller colonies, mostly mily and white colored ones. | Plates with Nutrient Only: no fungi, more colonies, smaller colonies, mostly mily and white colored ones. | ||
Plates with Nutrient and Antibiotic: fungi present, less colonies, larger colonies, no clear colored bacteria, pink and orange bacteria. | Plates with Nutrient and Antibiotic: fungi present, less colonies, larger colonies, no clear colored bacteria, pink and orange bacteria. | ||
When looking at the differences between the two plate types, I notice that the plates without the antibiotic have more abundant, smaller bacteria. Even though there still are baterica on the plates with antibiotic, there are not as many so it would be safe to assume in this case that the antibiotic and nutrient agar inhibits bacterial growth on the dish. However, based on my observations I would also suggest that the addition of the antibiotic allows for fungal growth which is not seen on the plates without tetracycline. Based on my dishes and my observations I would also say that orange and pink bacteria are unaffected by the tetracycline since they still appeared on those dishes. "Tetracycline works by binding specifically to the 30S ribosome of the bacteria, preventing attachment of the aminoacyl tRNA to the RNA-ribosome complex. It simultaneously inhibits other steps of the protein biosynthesis. Tetracycline can also alter the cytoplasmic membrane and this in turn causes leakage of nucleotides and other compounds out of the cell. This does not directly kill the bacteria but instead inhibit it." (quote from http://www.chm.bris.ac.uk/motm/tetracycline/antimicr.htm) | When looking at the differences between the two plate types, I notice that the plates without the antibiotic have more abundant, smaller bacteria. Even though there still are baterica on the plates with antibiotic, there are not as many so it would be safe to assume in this case that the antibiotic and nutrient agar inhibits bacterial growth on the dish. However, based on my observations I would also suggest that the addition of the antibiotic allows for fungal growth which is not seen on the plates without tetracycline. Based on my dishes and my observations I would also say that orange and pink bacteria are unaffected by the tetracycline since they still appeared on those dishes. "Tetracycline works by binding specifically to the 30S ribosome of the bacteria, preventing attachment of the aminoacyl tRNA to the RNA-ribosome complex. It simultaneously inhibits other steps of the protein biosynthesis. Tetracycline can also alter the cytoplasmic membrane and this in turn causes leakage of nucleotides and other compounds out of the cell. This does not directly kill the bacteria but instead inhibit it." (quote from http://www.chm.bris.ac.uk/motm/tetracycline/antimicr.htm) | ||
| Line 45: | Line 263: | ||
- gram negative | - gram negative | ||
- Diplobaccili | - Diplobaccili | ||
[[Image:]] | |||
[[Image:Screen_Shot_2014-02-16_at_3.29.33_PM.png]] | |||
Bacteria 3: | Bacteria 3: | ||
| Line 54: | Line 273: | ||
- gram negative | - gram negative | ||
- Staphylococcus (same as Bacteria 1 just more cells) | - Staphylococcus (same as Bacteria 1 just more cells) | ||
[[Image:Screen_Shot_2014-02-16_at_3.29.52_PM.png]] | |||
ATL | |||
Latest revision as of 08:32, 28 March 2014
March 22 : Embryology and Zebrafish Development
The objectives of this lab were to learn the stages of embryonic development, compare embryonic development in different organisms, and set up an experiment to study how environmental conditions affect embryonic development. Three procedures, each focusing on observing a different animals development (starfish, frog and chick) are going to aid in accomplishing the three objectives above. This lab will also feature the results of the Zebrafish experiment my group did focusing on salinity.
Table 1 Comparison of Embryological Features of a Developing Sea Star, Frog, and Chick
- no ocular micrometer for sea star
Table 2 Comparison of Ecological Aspects in Starfish, Frogs, and Chick
Zebrafish Experiment : This was carried out over a two week period where we tested the effects of salinity on developing embryos of zebrafish. There was definitely a more noticeable difference in the effects of the salinity on the ebryos the higher the concentration of salt there was in the petri dish. Zebrafish were chosen because of their ability to reproduce and how easy it is to keep track of what you are doing to them. Also their embryos are transparent, making it easier to see what changes are happening in the embryo while at the same time being able to absorb whatever is in the water they are developing in very easily. It should also be noted that on Day three of observation we needed to make new concentrations due to the fact that all of the zebrafish exposed to the 3% salinity for the first two days had died only a few hours after we put them in a petri dish. The new concentrations we made were 3% salinity, 0.3% salinity, and 0.03% salinity. Below is the raw data from the experiment we conducted.
Methods:
Day 1: On day one we set up our experiment by randomly selecting 40 embryos and evenly placing them in two different petri dishes. One had deerpark water in it while the other had a concentration of 3% NaCl. Once set up and the embryos were staged we were able to finish for the day after making our observations.
Day 3: We needed to make new concentrations due to the fact that all of the zebrafish exposed to the 3% salinity for the first two days had died only a few hours after we put them in a petri dish. The new concentrations we made were 3% salinity, 0.3% salinity, and 0.03% salinity. We restaged all of the fish and then made observations again that are on the table below.
Day 5: We removed the dead fish embryos that we found and then added more water to the control and more concentrated saline solution to their respective petri dish. We staged the fish once more and then made our observations that are in the chart below.
Day 7: Once again we needed to remove the dead fish embryos and then replace the water as we did before. On day seven there were no longer any fish left with the 3% salinity concentration dish. The observations made on that day are in the results table below.
Day 9: Today was the first day that we started fixing dead fish embryos. We fixed the same amount of fish from each plate whether dead or alive so we could compare them later on in our study with one another. The water was replaced again with the appropriate concentration with the observation recorded below.
Day 11: At this point all of the 0.3% NaCl fish embryos had died so we fixed more fish and then proceeded to refill the petri dishes with their correct concentrations of NaCl. The staging results and observations are below.
Day 14: This was the last day of observation where we fixed the rest of our fish embryos and then cleaned up all of the materials used. We then were able to observe our fixed embryos which is also recorded below.
Results:
The below table shows the observations on our fixed slides of the zebrafish embryos
Discussion:
The most important observations and results were probably on day three and day eleven / fourteen. Day three was significant because this was when we needed to reset our entire experiment since our testing group had all died and add some variables so that we could actually make some observations to determine what happens when an embryo is developing in that environment. Day eleven / fourteen was interesting as well since a lot of the control died off suddenly but we attribute this to a lack of nutrients since the majority of the class also experienced this same drop off. We also noticed that the embryos in control started to have greenish black eye pigmentation where the other embryos in testing group only had black pigment in their eyes. Obviously too much salt is not good for anybody but the amount of salt a human would have to consume to create a saline environment would be very high. I would say this study has more of an implication of fish who are developing in the incorrect environment than on actual people. Limitations would be the amount of time and uncontrollable weather conditions throughout the experiment but in terms of improvements or suggestions I think it would be interesting to see if these fish embryos can reproduce normally and lead a normal life even though the environments they developed in arent their natural ones.
ATL
February 26 : Mini Lab Entry (Mini Lab oriinally on February 26)
The objective of this lab was to analyze the results of the PCR that we did a couple weeks ago to deermine what bacteria is most prominent in our transects. Sample two in the chart below is my groups sample but the unknown sample is from the same transect but a different group because the first sample we tried to use PCR on did not work.
Table 1: Table of Results for PCR
ATL
February 26 : Fifth Lab Entry - Invertebrates (Lab 5 originally on February 12)
The main objectives of this lab are to uderstand the importance of invertebrates and to learn how sumple systems (including specialized cells and overall body plan) evolved into more complex systems. The following three procedures help accomplish these objectives.
Procedure 1: Observing Acoelmates, Pseudocoelmates, and Coelmates - In this procedure we were required to observe and describe the movements of the three types of worms and how the movement relates to their body structure. The first structure I observed was the nematode ascaris cross section which had no movement and is shown below (Figure 1). The second structure I observed was the nematode cephalobus whcih was the live section of the worm that had some movement (Figure 2). The last figure that we looked at for the nematode in this procedure was the coelmate cross section (Figure 3).
Next for this procedure we needed to observe planaria (flat worms) and do the same thing as we did for the earthworms above. The first image of planaria observed was an acoelmate cross section (Figure 4). Following that, I observed a planaria that was stained so i could see the digestive tract under the microscope (Figure 5). Lastly I observed the live section of the planaria and I noticed that it slides along the petri dish in order to move around (Figure 6).
Procedure 2: Analyzing the Invertebrates Collected in the Burlese Funnel - In this procedure we needed to observe any invertebrates that we found after using the Burlese funnel on our Hay Infusions. Unfortunately due to the weather leading up to when our samples were collected, all of the invertebrates that were in our transect were mostly washed away because of the location of the transect and the angle it is at. Due to this we needed to observe the west virginia samples that were provided incase something like this were to happen. We selected five of them and the following is what we observed.
Organism 1: Flea - 2 antennae, 6 legs, 2 body sections, ~3 mm
Organism 2: Fly - house fly, 2 wings, 2 body segments, ~5 mm
Organism 3: Fly - fruit fly, 2 wings, one body segment, ~2 mm
Organism 4: Termite - soft body, white, no wings, ~1 cm
Organism 5: Biting lice - 4 legs, no antennae, 2 body segments, no wings, ~2 mm
Procedure 3: Vertebrates and Niches - In this part we were asked to think of possible vertebrates that we might find in or around our transect. We were also asked to determine the classification of these along with identifying biotic and abiotic factors that would benefit each species mentioned. Finally we needed to make a food web of the organisms that we mentioned may appear at our transect. Five organisms that may be at my transect, the mini marsh, are the song sparrow, robin, chipmunk, raccoon, and the easter grey squirrel. Below is information on each of the organisms that may be found in my transect.
Song Sparrow:
-Phylum: Chordata
-Class: Aves
-Species: M. Melodia
-Genus: Melospiza
-Biotic: worms in soil are food
-Abiotic: water in transect, dead twigs and leaves for nests
Robin:
-Phylum: Chordata
-Class: Aves
-Species: T. Migratorious
-Genus: Turdus
-Biotic: worms in soil are food
-Abiotic: water in transect, dead twigs and leaves for nests
Chipmunk:
-Phylum: Chordata
-Class: Mammalia
-Species: T. Striatus
-Genus:Tamias
-Biotic: bulbs from plants in transect for food
-Abiotic: water in transect
Racoon:
-Phylum: Chordata
-Class: Mammilia
-Species: P. Lotor
-Genus: Procyon
-Biotic: the invertebrates for food that are in the transect
-Abiotic: water in transect
Eastern Grey Squirrell:
-Phylum: Chordata
-Class: Mammalia
-Species: S. Carolinensis
-Genus: Sciurus
-Biotic: seeds from transect as food
-Abiotic: water in transect, bark in transect for food
Food Web With Animals Relevant to Our Transect:
Robin and Song Sparrow --> Earthworms --> Bacteria and archaea --> Dead leaves
ATL
February 26 : Fourth Lab Entry - Plantae and Fungi (Lab 4 originally on Fecruary 5)
The main objectives of this lab were to undterstand the characteristics and diversity of plants, and to appreciate th function and importance of fungi. These objectives were accopmplished by the following five procedures that are discussed below.
Procedure 1 + 2: Collecting Five Plant Samples From the Transect, and Plant Vascularization - We needed to go over to our transect in front of Kogod School of Business and collect five different plant samples from the transect. Below is a table describing the different vegetation from our transect and all of the information about them that we were required to find. Also below is a picture of the transect so you can have a better understanding of where each of the plants were taken from. Procedure 2 asks for a description of the vascularization of each plant we found which is also in the table below.
Table 1: This is a table of the five plants we selected and the information about them and where they were found.
FIgure 1: This is a picture of the transect for a reference as to where we got our plants.
Procedure 3: Plant Specialization - In this procedure it was necessry for us to describe the shape, size and cluster arrangement of the leaves from each of the transect plants.
Plant #1: There were no leaves on the cat tail and based on the surrounding environment no evidence that there were leaves on the plant. Plant #2: The leaves on the tall light brown grass type plant were very small and narrow. They were also very numerous and were only on the ends of each stem. Plant #3: The third plant had broad leaves which were dark in color with visible veins. This was the red bush. Plant #4: The fourth plant had round green leaves in which the veins were visible. This was the green ground plant. Plant #5: The fifth plant was grass so it only had one leaf (the blade of grass) which was straight and narrow with no veins visible.
Procedure 4: Plant Reproduction - Next we needed to identify each of the plants we found in our transect as monocot or dicot. After observing each of the plants and their seeds or their buds if the had them, we were able to determine which of the two each of our plants were. All of the plants we looked at except for the grass, were dicot. The grass however, was monochot. We also observed the lily flowers provided in class and we determined those to be monocots.
Procedure 5: Observing Fungi - In this procedure we observed a fungi presented to us on a petri dish that we needed to observe. The fungi that we observed happened to be bread mold. We needed to determine the importance of fungi sporangia as well. I knew this was a fungus that I was looking at because I could see the mycellum and sporangia under the desiction scope and these are two things that you often find in fungi. The sporangia is important to fungi because it forms spores for the fungi, which is used for asexual reproduction in the fungi. Below is a picture of the bread mold. The black dots are the sporangia.
Figure 2: Bread mold
ATL
February 16 : Third Lab Entry - Microbiology and Identifying Bacteria with DNA (Lab 3 originally on January 29)
The main objectives of this lab were to understand the characteristics of bacteria, to observe antibiotic resistance, and to understand how DNA sequences are used to identify species. The three objectives that I have stated were accomplished by the following three procedures described below.
Procedure 1: Quantifying and Observing Microorganisms - We needed to first make another set of observations from our transects that we had made on the first lab of the semester. After we had done that, we then needed to observe the petri dishes that we had prepared the previous class from the serial dilutions of the Hay Culture. We needed to observe all seven dilution plates, four of which with a nutrient agar, and three of which with a nutrient and tetracycline (antibiotic) agar. For each petri dish we had to record the number of colonies in each dish and record them in a table. I do not think that we would find any archaea on the petri dishes since they only grow in harsh environments and our transects were not considered harsh environments. The appearance and smell of the transect may have changed week to week because new bacteria may be developing within, and things may be decomposing etc.
Hay Culture Observations: -some water evaporated -smell not as potent -water looks darker throughout -still some green shoots -debris is still on bottom -no section like division this time; one big mess in there
Petri Dish Observations: 10^-3 (nutrient only) : counted approximately 1940 colonies which converts to approximately 1,940,000 colonies per ml 10^-5 (nutrient only) : counted approximately 150 colonies which converts to approximately 15,000,000 colonies per ml 10^-7 (nutrient onliy) : counted approximately 6 colonies which converts to approximately 60,000,000 colonies per ml 10^-9 (nutrient only) : counted approximately 0 colonies which converts to approximately 0 colonies per ml 10^-3 (nutrient and tet) : counted approximately 50 colonies which converts to approximately 50,000 colonies per ml 10^-5 (nutrient and tet) : counted approximately 3 colonies which converts to approximately 300,000 colonies per ml 10^-7 (nutrient and tet): counted approximately 0 colonies which converts to approximately 0 colonies per ml
Procedure 2: Antibiotic Resistance - In this procedure we were required to observe our petri dishes once again along with our recorded table in order to make comments about the appearances and differences in between the plates with nutrient agar and the plates with nutrient and antibiotic agar. After observing all of the collected information, my group noticed the following:
Plates with Nutrient Only: no fungi, more colonies, smaller colonies, mostly mily and white colored ones.
Plates with Nutrient and Antibiotic: fungi present, less colonies, larger colonies, no clear colored bacteria, pink and orange bacteria.
When looking at the differences between the two plate types, I notice that the plates without the antibiotic have more abundant, smaller bacteria. Even though there still are baterica on the plates with antibiotic, there are not as many so it would be safe to assume in this case that the antibiotic and nutrient agar inhibits bacterial growth on the dish. However, based on my observations I would also suggest that the addition of the antibiotic allows for fungal growth which is not seen on the plates without tetracycline. Based on my dishes and my observations I would also say that orange and pink bacteria are unaffected by the tetracycline since they still appeared on those dishes. "Tetracycline works by binding specifically to the 30S ribosome of the bacteria, preventing attachment of the aminoacyl tRNA to the RNA-ribosome complex. It simultaneously inhibits other steps of the protein biosynthesis. Tetracycline can also alter the cytoplasmic membrane and this in turn causes leakage of nucleotides and other compounds out of the cell. This does not directly kill the bacteria but instead inhibit it." (quote from http://www.chm.bris.ac.uk/motm/tetracycline/antimicr.htm)
Procedure 3: Bacteria Cell Morphology Observations - In this procedure we had to observe different bacteria from our agar plates that we chose after mounting them with oil. We also had to make a gram stain from our different bacteria that we cultured from our agar plates. We were required to record our observations of what three bacteria looked like, which agar plate they came from, what they looked like before we put them under the microscope, number of colonies, their motility and shape, and whether they were gram negative or positive. Below is what we observed.
Bacteria 1:
- 10^-3 tet plate
- pink, tear drop shape, convex, smooth
- #colonies: about 50,000
- cocci, smushed together, tetrads, no movement
- gram positive
- Staphylococcus

Bacteria 2: - 10^-7 nutrient plate - circular, flat with distinct circle in middle, dark milky color, not really that smooth - #colonies: about 60,000,000 - cocci, staphylococcus, and streptococcus, no movement - gram negative - Diplobaccili
Bacteria 3: - 10^-5 nutrient plate - orange, circular, convex, smooth - #colonies: about 300,000 - cocci, staphylococcus, no movement - gram negative - Staphylococcus (same as Bacteria 1 just more cells)
ATL
February 8 : Second Lab Entry - Identifying Algae and Protists (Lab 2 originally on January 22)
The main objectives of this lab were to understand how to use a dichotomos key and the characteristics of algae and protists. These two objectives were accomplished by completing the two procedures described below.
Procedure 1: How to Use a Dichotomous Key - We needed to be able to look at two samples that we had made wetmounts of in and identify two different types of organisms on each wet mount. We were required to describe what each organism looked like, how big it was, and then identify what it could be by using a dichotomous key. Based on the many questions and images that are available on the dichotomous key, I felt fairly confident that my guesses were correct.
Wet Mount #1:
Organism 1: - long slim, blue-green color - size: 1500 µl - Stentor
Organism 2: - large, creeps using pseudopodia, many small nuclei - size: 150 µl - Pelomyxa
Wet Mount #2:
Organism 1: - long slim, pinkish-rose color - size: 2500 µl - Blepharisma
Organism 2: - oval shaped, contracting w/ lots of nuclei, barely moving - size: 50 µl - Gonium
Procedure 2: Hay Infusion Culture Observations - In this section of the lab me and my transect group needed to observe the transect that we made the previous lab and record what we saw and then observe wet mounts taken from the Hay Infusion to see what organisms were present. We needed to observe three different organisms from each wet mount (we made two). We decided to make a wet mount of the bottom of the Hay Culture, and then a second wet mount from the top of the Hay Culture. Organisms near plant matter could possibly be photosynthetic in nature as opposed to those not near the plants. I would predict that in a couple months the small abount of green growth that was observed would probably become more abundant along with the amount of algae and bacteria that were in our samples. Carrying capacity was definitely a player in the culture because there is no way that more than the maximum amount of organisms possible for that niche size can exist in that environment. If we had observed one of the plants that were in our culture, we would have most definitely been able to identify it as something alive because it can do photosynthesis to get its energy, it is made up of cells, it has genetic information in the nuclei of its cells, the cells can replicate, and they are a product of evolution, most likely evolving from green algae. Below is what we observed / found.
Appearance of Hay Culture: - smells moldy (kind of like a seafood smell) - top is thick with some kind of algae maybe? - sprout of green growth / green shoot on top part of culture - the water is a brownish color - nothing really floating in the middle of the culture - sedement on bottom - white type of filmy liquid is right under the water surface
Top Sample Wet Mount:
Organism 1:
- no color, oval shaped, has many leg looking things moving it
- size: 62.5 µl
- Euplotes

Organism 2:
- circular shape, green, many cells inside
- size: 25 µl
- Gonium

Organism 3:
- colorless, oval shaped, small
- size: 25 µl
- Amoeba

Bottom Sample Wet Mount:
Organism 1:
- reddish, long and slim
- size: 800 µl
- Blepharisma

Organism 2:
- colorless, oval shaped, small
- size: 20 µl
- Amoeba
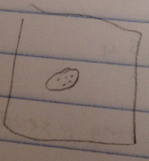
Organism 3:
- brown looking, small, not moving
- size: 12.5 µl
- Arcella

2/6/14, lab 1 notes
Great work! Some notes: -Make sure you include pics before Sunday -In the future, weave the answers to the questions into the introduction or discussion section. Do not write question and respond with answer. -Start working on building a map of your transect to detail your land and where your samples are taken from. We will talk about this more Wednesday
Great job!! AP
January 29: First Lab Entry - Biological Life at AU (Lab 1 originally on January 15)
The main objectives of this lab were to understand natural selection and to understand the abiotic and biotic characteristics of a niche. These objectives were accomplished with the two procedures described below.
Procedure 1: The Volvocine Line - We needed to identify the different members of the valvocine line for this procedure and specify the number of cells, colony size, and its reproductive specialization for each of the different members. I predicted that the more advanced along the valvocine line we moved, the more complex what I saw would become. I looked at each of the three options on wet mounts under a microscope using the 4x, 10x, or 40x objective lens in order to figure out the information asked of me. Based on the data collected I was correct in assuming that the genera would become more complex over time. I was able to address the question posed in the beginning of this procedure.
Chlamydomonas: Unicellular, colony size was 7.5µl, isogamy.
Gonium: Eight cells, colony size was 60µl, isogamy.
Volvox: Too many cells to count, all appear to be in a bulb shape, colony size was 125µl, oogamy.
-What is the significance of cell specialization across these three genera? Natural selection is shown through the changes in complexity of these three genera over time.
-Does evolution always move towards increased complexity? Provide an example. No I dont think it necessarily does. People originally have tails which are considered vestigial traits that go away the more they develop in the womb. The lack of tail makes us less complex than if we were to be born with tails.
Procedure 2: Defining a Niche at AU - We were put into groups of three and were sent to observe a transect here at AU. We also were given a 50ml conical tube to collect a soil/surface plant sample to bring back to class to use for the next few labs. In this lab we were asked to record our observations in a notebook to explain what our transect looked like. Once we returned to the lab we needed to make our Hay Infusion Culture by adding 500ml of water and .1g of dried milk to 10-12g of our sample and then close and leave it for next class.
Observations of Transect: (Located in front of Kogod)
Abiotic: Small rocks, larger rocks, big boulders, water, sun, soil
Biotic: Moss, cat tails, grass, weeds, red bark shrub, algae, bacteria, fungi, protists
Also noticed dead bark and leaves. Water runs downhill through transect.
ATL
January 22
Successfully entered text
ATL


















