User:Allison K. Alix/Notebook/Thesis Research/2013/04/09: Difference between revisions
No edit summary |
|||
| (9 intermediate revisions by the same user not shown) | |||
| Line 1: | Line 1: | ||
{|{{table}} width="800" | {|{{table}} width="800" | ||
|- | |- | ||
|style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> | |style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> Reacting Ethane thiol with AuNP</span> | ||
|style="background-color: #F2F2F2" align="center"|<html><img src="/images/9/94/Report.png" border="0" /></html> [[{{#sub:{{FULLPAGENAME}}|0|-11}}|Main project page]]<br />{{#if:{{#lnpreventry:{{FULLPAGENAME}}}}|<html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>[[{{#lnpreventry:{{FULLPAGENAME}}}}{{!}}Previous entry]]<html> </html>}}{{#if:{{#lnnextentry:{{FULLPAGENAME}}}}|[[{{#lnnextentry:{{FULLPAGENAME}}}}{{!}}Next entry]]<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html>}} | |style="background-color: #F2F2F2" align="center"|<html><img src="/images/9/94/Report.png" border="0" /></html> [[{{#sub:{{FULLPAGENAME}}|0|-11}}|Main project page]]<br />{{#if:{{#lnpreventry:{{FULLPAGENAME}}}}|<html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>[[{{#lnpreventry:{{FULLPAGENAME}}}}{{!}}Previous entry]]<html> </html>}}{{#if:{{#lnnextentry:{{FULLPAGENAME}}}}|[[{{#lnnextentry:{{FULLPAGENAME}}}}{{!}}Next entry]]<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html>}} | ||
|- | |- | ||
| Line 13: | Line 13: | ||
==Procedures== | ==Procedures== | ||
Part 1: Preparation of 2.98μM ethane thiol | '''Part 1: Preparation of 1mL of 2.98μM ethane thiol''' | ||
WARNING: ethanethiol is very volatile. Handle under the hood only wearing PPE. | |||
* original concentration of AuNP = 19.4nM | |||
* desired concentration of ethanethiol = 19.4nM x(15393) = 298624.2nM = 298μM | |||
* density of ethanethiol = 0.839g/mL | |||
1) Find the concentration of 0.0125mL pure ethanethiol in 5mL water | |||
0.839g/mL x 0.0125mL = 0.0104875g | |||
0.0104875g/(62.13 g/mol) = 1.68799 x 10<sup>-4</sup> mol | |||
1.68799 x 10<sup>-4</sup> mol/5mL x (1000mL/1L) = 0.034M = 34mM | |||
340mM (x) = (30mM)(1mL) | |||
x = 0.088mL = 88μL in 912μL H<sub>2</sub>O | |||
30mM (x) = 3mM (1mL) | |||
x = 100μL in 900μL | |||
3mM(x) = 298μM (1mL) | |||
x = 99μL in 901μL to obtain 298μM ethanethiol | |||
2) Reacting AuNP with ethanethiol | |||
* react 200μL 298μM ethane thiol with 200μL 19.4nM AuNP (synthesized by Dr. Miller) | |||
'''Part 2: Measure absorbance/fluorescence of AuNP-MB''' | |||
'''Part 3: Measure absorbance/fluorescence of AuNP-ThT''' | |||
==Data== | |||
Part 2: | |||
[[Image:ThiolDNA_MB_pic_UV-Vis.png]] | |||
UV-Vis Spectrum of thiol-DNA/MB hybrid | |||
[[Image:ThiolDNA_MB_pic.png]] | |||
Fluorescence Spectrum of thiol-DNA/MB hybrid | |||
Part 3: | |||
[[Image:ThiolDNA_ThT_pic_UV-Vis.png]] | |||
UV-Vis Spectrum of thiol-DNA/ThT hybrid | |||
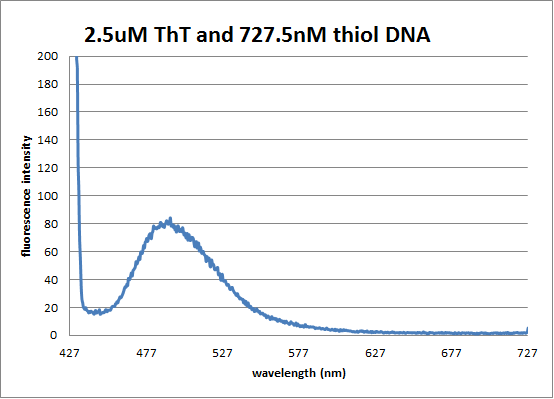
[[Image:ThiolDNA ThT pic.png]] | |||
Fluorescence Spectrum of thiol-DNA/ThT hybrid | |||
Revision as of 08:19, 12 April 2013
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |
Objectives
ProceduresPart 1: Preparation of 1mL of 2.98μM ethane thiol WARNING: ethanethiol is very volatile. Handle under the hood only wearing PPE.
1) Find the concentration of 0.0125mL pure ethanethiol in 5mL water 0.839g/mL x 0.0125mL = 0.0104875g 0.0104875g/(62.13 g/mol) = 1.68799 x 10-4 mol 1.68799 x 10-4 mol/5mL x (1000mL/1L) = 0.034M = 34mM
340mM (x) = (30mM)(1mL) x = 0.088mL = 88μL in 912μL H2O 30mM (x) = 3mM (1mL) x = 100μL in 900μL 3mM(x) = 298μM (1mL) x = 99μL in 901μL to obtain 298μM ethanethiol 2) Reacting AuNP with ethanethiol
Part 2: Measure absorbance/fluorescence of AuNP-MB Part 3: Measure absorbance/fluorescence of AuNP-ThT DataPart 2: UV-Vis Spectrum of thiol-DNA/MB hybrid Fluorescence Spectrum of thiol-DNA/MB hybrid Part 3: UV-Vis Spectrum of thiol-DNA/ThT hybrid Fluorescence Spectrum of thiol-DNA/ThT hybrid
| |