User:Alicia Rasines Mazo/Notebook/CHEM-581 Experimental Chemistry I/2014/09/19: Difference between revisions
From OpenWetWare
No edit summary |
(fix raw html notebook nav) |
||
| (10 intermediate revisions by one other user not shown) | |||
| Line 2: | Line 2: | ||
|- | |- | ||
|style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> Project name</span> | |style="background-color: #EEE"|[[Image:owwnotebook_icon.png|128px]]<span style="font-size:22px;"> Project name</span> | ||
|style="background-color: #F2F2F2" align="center"| | |style="background-color: #F2F2F2" align="center"|[[File:Report.png|frameless|link={{#sub:{{FULLPAGENAME}}|0|-11}}]][[{{#sub:{{FULLPAGENAME}}|0|-11}}|Main project page]]<br />{{#if:{{#lnpreventry:{{FULLPAGENAME}}}}|[[File:Resultset_previous.png|frameless|link={{#lnpreventry:{{FULLPAGENAME}}}}]][[{{#lnpreventry:{{FULLPAGENAME}}}}{{!}}Previous entry]] }}{{#if:{{#lnnextentry:{{FULLPAGENAME}}}}|[[{{#lnnextentry:{{FULLPAGENAME}}}}{{!}}Next entry]][[File:Resultset_next.png|frameless|link={{#lnnextentry:{{FULLPAGENAME}}}}]]}} | ||
|- | |- | ||
| colspan="2"| | | colspan="2"| | ||
| Line 10: | Line 10: | ||
* To finish analysing PVA-Malachite green data | * To finish analysing PVA-Malachite green data | ||
* To start analysis on X-ray | * To start analysis on X-ray | ||
*To | * To complete preparation of ionic liquid modified clay | ||
* To set up experiments for the measurement of absorbance of Mercury and Arsenic by films | |||
===New film synthesis=== | ===New film synthesis=== | ||
* 1.0022 g 22,000 MW PVA were used to make the PVA polymer film | * 1.0022 g 22,000 MW PVA were used to make the PVA polymer film | ||
* 1.0044 g 22,000 MW PVA and 0.1104 g Na Montmorillonite were used for the synthesis of 10% w/w PVA-Clay polymer film. | * 1.0044 g 22,000 MW PVA and 0.1104 g Na Montmorillonite were used for the synthesis of 10% w/w PVA-Clay polymer film. | ||
===Completing preparation of ionic liquid modified clay=== | |||
Continued from [http://openwetware.org/wiki/User:Alicia_Rasines_Mazo/Notebook/CHEM-581_Experimental_Chemistry_I/2014/09/17 Set. 17 2014] | |||
#Ground with mortar | |||
#Stored in dissecator | |||
===Measuring the Absorbance of Mercury and Arsenic=== | ===Measuring the Absorbance of Mercury and Arsenic=== | ||
| Line 21: | Line 27: | ||
| align="center" style="background:#f0f0f0;"|'''Solution''' | | align="center" style="background:#f0f0f0;"|'''Solution''' | ||
| align="center" style="background:#f0f0f0;"|'''Empty glass vial (g)''' | | align="center" style="background:#f0f0f0;"|'''Empty glass vial (g)''' | ||
| align="center" style="background:#f0f0f0;"|'''Glass Vial + | | align="center" style="background:#f0f0f0;"|'''Glass Vial + film (g)''' | ||
| align="center" style="background:#f0f0f0;"|'''Film weight (g)''' | | align="center" style="background:#f0f0f0;"|'''Film weight (g)''' | ||
|- | |- | ||
| Line 65: | Line 71: | ||
* Note: in order to make up the specified dilutions (Table above) of As and Hg, dilutions of stock solutions were prepared. | * Note: in order to make up the specified dilutions (Table above) of As and Hg, dilutions of stock solutions were prepared. | ||
* | * Eleni prepared the arsenic dilutions | ||
*Madeleine | * James and Madeleine prepared the mercury dilutions | ||
*Dilutions: | *Dilutions: | ||
| Line 83: | Line 89: | ||
*#(268 ppm)(V)=(50 ppm)(50mL) | *#(268 ppm)(V)=(50 ppm)(50mL) | ||
*#*9.33mL in 50mL volumetric | *#*9.33mL in 50mL volumetric | ||
10 mL of each solution were poured into glass vials containing the PVA-polymer films | |||
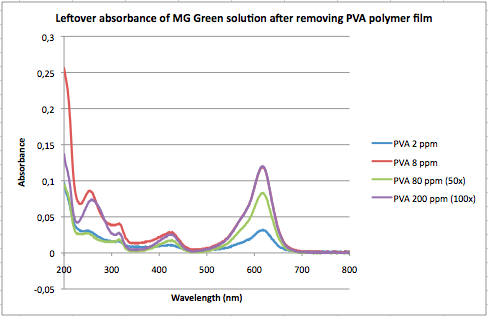
===Second Analysis of UV-Vis MG data for PVA-polymer film=== | ===Second Analysis of UV-Vis MG data for PVA-polymer film=== | ||
[[Image:MGPVAUVVis 190914.png]] <br.> | |||
Used MG calibration curves obtained on [http://openwetware.org/wiki/User:Alicia_Rasines_Mazo/Notebook/CHEM-581_Experimental_Chemistry_I/2014/09/05 Sept. 5 2014] | Used MG calibration curves obtained on [http://openwetware.org/wiki/User:Alicia_Rasines_Mazo/Notebook/CHEM-581_Experimental_Chemistry_I/2014/09/05 Sept. 5 2014] | ||
*ε<sub>615nm</sub>=0.1293 M<sup>-1</sup>cm<sup>-1</sup> | *ε<sub>615nm</sub>=0.1293 M<sup>-1</sup>cm<sup>-1</sup> | ||
| Line 113: | Line 120: | ||
|} | |} | ||
Note that 10 of MG solution were added per vial, so that 2 ppm MG = (1.760 mg MG/L)×0.010L=0.0176 mg MG/film <-- absorbed amount <br.> | Note that 10 of MG solution were added per vial, so that 2 ppm MG = (1.760 mg MG/L)×0.010L=0.0176 mg MG/film <-- absorbed amount <br.> | ||
The weight of the film submerged in 2 ppm MG solution was 0.1060 g. Refer to | The weight of the film submerged in 2 ppm MG solution was 0.1060 g. Refer to [http://openwetware.org/wiki/User:Alicia_Rasines_Mazo/Notebook/CHEM-581_Experimental_Chemistry_I/2014/09/12 Sept. 12 2014] for further film weights. | ||
*(0.0176mg MG/0.01060g film)='''0.0166 mg MG/g film''' | |||
*8 ppm: '''0.728 mg MG/g film''' | |||
*80 ppm: '''4.49 mg MG/g film''' | |||
*200 ppm: '''10.8 mg MG/g film''' | |||
===Measuring amount of MG absorbed onto glass vial=== | |||
[[Image:UVVIS Controls 190914.jpeg]]<br.> | |||
As in previous section, the molar absorptivity and calibration curve from Sept. 5 2014 were used. <br.> | |||
80 ppm MG in glass vial control: 42.87 ppm were absorbed by glass (Calculations as show in previous section) | |||
*'''0.429 mg MG/glass vial''' | |||
200 ppm MG in glass vial control: 88.63 ppm were absorbed by glass | |||
*'''0.886 mg MG/glass vial''' | |||
<!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit below this line unless you know what you are doing. ##### --> | ||
__NOTOC__ | __NOTOC__ | ||
Latest revision as of 00:19, 27 September 2017
Tasks for 19 September
New film synthesis
Completing preparation of ionic liquid modified clayContinued from Set. 17 2014
Measuring the Absorbance of Mercury and Arsenic
10 mL of each solution were poured into glass vials containing the PVA-polymer films Second Analysis of UV-Vis MG data for PVA-polymer film
Solving for concentration:
Note that 10 of MG solution were added per vial, so that 2 ppm MG = (1.760 mg MG/L)×0.010L=0.0176 mg MG/film <-- absorbed amount <br.> The weight of the film submerged in 2 ppm MG solution was 0.1060 g. Refer to Sept. 12 2014 for further film weights.
Measuring amount of MG absorbed onto glass vial
200 ppm MG in glass vial control: 88.63 ppm were absorbed by glass
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||