Todd:Pictet-Spengler to PZQ: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
No edit summary |
No edit summary |
||
| Line 28: | Line 28: | ||
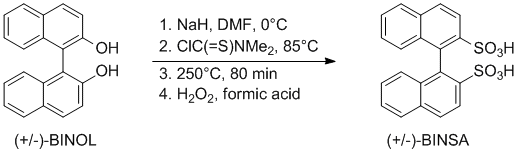
'''1,1-Binaphthyl-2,2-disulfonate''' | '''1,1-Binaphthyl-2,2-disulfonate''' | ||
[[Image:Binaphtyl-disulfonic acid procedure.gif]] | |||
1. Step: [http://www.ourexperiment.org/racemic_pzq/567 Preparation of 1,1’-Binaphthalene-2,2’-diyl-O,O’-bis(N,N’-dimethylthiocarbamate) (MW45-1)] | 1. Step: [http://www.ourexperiment.org/racemic_pzq/567 Preparation of 1,1’-Binaphthalene-2,2’-diyl-O,O’-bis(N,N’-dimethylthiocarbamate) (MW45-1)] | ||
Revision as of 23:49, 24 May 2011
Pictet-Spengler route to Praziquantel
Michael Woelfle,1 and Matthew H. Todd1*
1. School of Chemistry, The University of Sydney, NSW 2006, Australia
- To whom correspondence should be addressed: Tel. +61 2 9351 2180, matthew.todd@sydney.edu.au
Abstract
The Pictet-Spengler route to Praziquantel
The PZQ precursor
By using the Ugi reaction
Introduction
Results
Preparation of the Ugi-intermediates
Synthesis of the catalysts
1,1-Binaphthyl-2,2-disulfonate
1. Step: Preparation of 1,1’-Binaphthalene-2,2’-diyl-O,O’-bis(N,N’-dimethylthiocarbamate) (MW45-1)
2. Step: Preparation of 1,1’-Binaphthalene-2,2’-diyl-S,S’-bis(N,N’-dimethylthiocarbamate) (MW45-2)
3. Step: Preparation of 1,1’-Binaphthalene-2,2’-disulfonic acid (MW45-3)
- Pyridinium 1,1′-Binaphthyl-2,2′-disulfonates as Highly Effective Chiral Brønsted Acid−Base Combined Salt Catalysts for Enantioselective Mannich-Type Reaction, M. Hatano, T. Maki, K. Moriyama, M. Arinobe and K. Ishihara, J. Am. Chem. Soc. 2008, 130, 16858–16860; DOI: 10.1021/ja806875c.
- A Powerful Chiral Counteranion Motif for Asymmetric Catalysis, P. García-García, F. Lay, P. García-García, C. Rabalakos, B. List, Angew. Chem. Int. Ed. 2009, 48, 4363 –4366; DOI: 10.1002/anie.200901768.
References
- Synthesis of Praziquantel, J. H. Kim, Y. S. Lee, H. Park and C. S. Rim, Tetrahedron , 1998, 54, 7395-7400. (DOI: doi:10.1016/S0040-4020(98)00401-3)