Todd:Catalytic, Asymmetric Pictet-Spengler Reaction: Difference between revisions
| Line 112: | Line 112: | ||
Iso-Pictet-Spengler (C3 of indole) [http://dx.doi.org/10.1021/ol202300t Jacobsen 2011]<br> - MNR Done | Iso-Pictet-Spengler (C3 of indole) [http://dx.doi.org/10.1021/ol202300t Jacobsen 2011]<br> - MNR Done | ||
Thiourea plus a proton: [http://dx.doi.org/10.1021/ol802887h Jacobsen 2009] - MNR Done | Thiourea plus a proton: [http://dx.doi.org/10.1021/ol802887h Jacobsen 2009] - MNR Done | ||
Revision as of 16:25, 10 January 2012
The Catalytic, Asymmetric Pictet-Spengler Reaction
Katrina Badiola, School of Chemistry, The University of Sydney, NSW 2006, Australia
Murray N. Robertson, School of Chemistry, The University of Sydney, NSW 2006, Australia
Matthew H. Todd, School of Chemistry, The University of Sydney, NSW 2006, Australia
Additional authors - add alphabetically if you contribute something substantial (e.g., the summary of a paper with a scheme). Final arbitration on authorship (as opposed to acknowledgement) lies with Mat Todd
(This article is intended as a stand-alone review. It also acts as background to the open science project to find a catalytic, asymmetric route to praziquantel. The review is open source, meaning anyone can add and edit. When it is deemed to be up to date, comprehensive, error-free and well-written, it will be submitted for publication to a peer-reviewed open access journal, but editing can continue here after that point. This page is currently active - when this changes <= these words will be changed (and you can see when the last edit of this page was made at the bottom). References for this page may be found in full at the Mendeley page). If you want to get in touch to ask questions please do not use email. You can use the talk page (tab above), or directly insert a question on this page (below) with your initials, or discuss via Google+ pages: Mat, (please add other public places where you can be contacted if you contribute as an author).
Important note on simultaneous edits: If you are intending to work for some time on editing the page, we'd recommend writing text elsewhere then pasting it in here, since there is a small but non-zero chance that you might simultaneously edit the same section as someone else, resulting in the chance of the loss of some information.
Schemes: Use Wiley/Angewandte settings for the .cdx files and add below as .png files.
Introduction
Importance of the structural motifs constructed with the reaction:
- Brown, R. T. In Indoles; Saxton, J. E., Ed.; Wiley- Interscience: New York, 1983; Part 4 (The Monoterpenoid Indole Alkaloids)
- Bentley, K. W. Nat. Prod. Rep. 2004, 21, 395-424 and references therein
Racemic/Achiral
To Do:
- Pictet, A.; Spengler, T. Ber. Dtsch. Chem. Ges. 1911, 44, 2030-2036.
- Tatsui, G. J. Pharm. Soc. Jpn. 1928, 48, 92.
- Cox, E. D.; Cook, J. M. Chem. Rev. 1995, 95, 1797- 1842
- Chrzanowska, M.; Rozwadowska, M. D. Chem. Rev. 2004, 104, 3341-3370.
- Nature: Naoi, M.; Maruyama, W.; Nagy, G. M. Neurotoxicology 2004, 25, 193- 204
Diastereoselective
To Do:
- Cox, E. D.; Hamaker, L. K.; Li, J.; Yu, P.; Czerwinski, K. M.; Deng, L.; Bennett, D. W.; Cook, J. M. J. Org. Chem. 1997, 62, 44-61 and references therein.
- Czarnocki, Z.; MacLean, D. B.; Szarek, W. A. Can. J. Chem. 1986, 64, 2205-2210.
- Czarnocki, Z.; Suh, D.; MacLean, D. B.; Hultin, P. G.; Szarek, W. A. Can. J. Chem. 1992, 70, 1555-1561.
- Czarnocki, Z.; Mieckzkowsi, J. B.; Kiegiel, J.; Arazn ́y, Z. Tetrahyedron: Asymmetry 1995, 6, 2899-2902.
- Waldmann, H.; Schmidt, G.; Henke, H.; Burkard, M. Angew. Chem., Int. Ed. Engl. 1995, 34, 2402-2403.
- Schmidt, G.; Waldmann, H.; Henke, H.; Burkard, M. Chem. Eur. J. 1996, 2, 1566-1571.
- Gremmen, C.; Willemse, B.; Wanner, M. J.; Koomen, G.-J. Org. Lett. 2000, 2, 1955-1958.
- Gremmen, C.; Wanner, M. J.; Koomen, G.-J. Tetrahedron Lett. 2001, 42, 8885-8888.
- Tsuji, R.; Nakagawa, M.; Nishida, A. Tetrahedron: Asymmetry 2003, 14, 177- 180.
Enantioselective
Lewis Acids
To Do:
- Yamada, H.; Kawate, T.; Matsumizu, M.; Nishida, A.; Yamaguchi, K.; Nakagawa, M. J. Org. Chem. 1998, 63, 6348-6354
- Hino, T.; Nakagawa, M. Heterocycles 1998, 49, 499-531.
Bronsted Acids
List reported the first Bronsted acid-catalyzed enantioselective Pictet-Spengler reaction in 2006 (10.1021/ja057444l). Chiral, substituted phosphoric acids were shown to be effective in the PS cyclization of tryptamines with a number of aliphatic and aromatic aldehydes (Scheme List 2006). The diester functionality was found to be necessary, presumably due to promotion of a clean reaction through the Thorpe-Ingold effect (and an aldol side reaction was observed when the esters were absent). Lower yields were typically observed when the methoxy group was absent from the tryptamine aromatic ring.

In 2007, Hiemstra reported the enantioselective synthesis of tetra-β-carbolines via the in situ formation of N-sulfenyliminium ions (10.1002/anie.200701808). Stabilization of the intermediate iminium by the N-tritylsulfenyl group was effective at promoting the acid-catalyzed PS reaction by substituted enantiopure BINOL phosphoric acids. Addition of a radical scavenger prevented decomposition of the resulting N-tritylsulfenyltetrahydro-β-carbolines by homolytic cleavage. A variety of substituted tetra-β-carbolines were prepared in the one-pot reaction with high yield and high ee.

A procedure for the synthesis of chiral N-benzyl-protected tetrahydro-β-carbolines was outlined by Hiemstra in 2008 (10.1021/jo8010478). The irreversible enantioselective PS reaction of N-benzyltryptamine with various aliphatic and aromatic aldehydes was catalyzed, once more, by the potent enantiopure substituted phosphoric acids. Up to 100% conversion and high ee (78-85%) was observed in the case of the triphenylsilyl substituted acid. Of the aliphatic aldehydes, no product was observed with the easily enolisable phenylacetaldehyde and low ee (8%) was obtained with 3-phenylpropanal.
The yield and ee of both procedures were optimised removal of water by molecular sieves, possibly due to reduced decomposition of the acid-precursor complexes by water. (<-- need to fix this sentence).
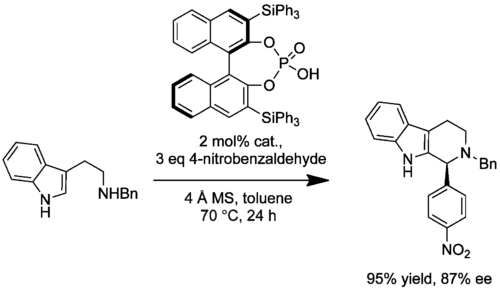
The preparation of chiral tetrahydro-β-carbolines was exploited in the total syntheses of (-)-arboricine and (+)-yohimbine, reported by Hiemstra in 2008 (10.1021/ol900888e) and 2011 (10.1021/jo201657n). The key enantioselective step in both syntheses were asymmetric Pictet-Spenlger reactions catalysed by enantiopure phosphoric acids, which dictated the stereochemical selectivity of subsequent reactions. In the PS reaction for the synthesis of (-)-arboricine, implementation of the dioxolane protecting group prevented hemiaminal formation of the ketone with the indole nitrogen, increasing the yield for the reaction. Interestingly, the dioxolane facilitated the PS reaction by improving ee and increasing the rate of the reaction. The highest ee (89%) was obtained using the H8-BINOL phosphoric acid (slightly greater steric hinderance than the BINOL phosphoric acid). A similar approach was adopted in the synthesis of (+)-yohimbine. Tautomerisation of the intermediate iminium ion to the unreactive, conjugate enamine was avoided by substituting the obvious(?) β,γ-unsaturated aldehyde with the phenylseleno aldehyde. (ALMOST FINISHED, KAB).


The enantioselective potency of chiral phosphoric acids in catalytic Mannich-type reactions were independently reported by Akiyama (10.1002/anie.200353240) and Terada (10.1021/ja0491533) in 2004. A number of substituted enantiopure BINOL phosphoric acids were screened by the two groups and both found that aromatic β-substitution of the BINOL acids increased the enantioselectivity of the catalysts. (Not sure where this should go, or which aspect to focus on, KAB) Terada et al (J. Am. Chem. Soc. 2004, 126, 5356-5357) (10.1021/ja0491533) - Summary of summary (TBC 11/01/12): Evaluation of chiral phosphoric acids (CPA) in direct Mannich-type reactions. Synthesis of beta-aminoketone. Advantageous properties of CPA include tetradentate P(V) preventing rotation by forming ring structure (not formed in carboxylic or sulfonic acids). Acidity. Lewis base site at phosphoric oxygen = bifunctional catalyst. Extension of the aromatic substitution to the para direction improved enantioselectivity (up to 95% ee). Para and ortho-substituted. Determined absolute configuration reacting to phenylglycine (conserved chirality?) - Still working on this, KAB Terada (b)

To Do:
Early work on addition reactions to aldimines catalyzed by binaphthol-derived chiral phosphoric acids: Akiyama et al ((a) Angew. Chem., Int. Ed. 2004, 43, 1566-1568; (b) Org. Lett. 2005, 7, 2583-2585; (c) Akiyama, T. PCT Int. Appl. WO 200409675, 2004; (d) Adv. Synth. Catal. 2005, 347, 1523-1526. And Terada et al ((a) J. Am. Chem. Soc. 2004, 126, 5356-5357. (b) J. Am. Chem. Soc. 2004, 126, 11804-11805. (c) J. Am. Chem. Soc. 2005, 127, 9360-9361; (d) Terada, M.; Uraguchi, D.; Sorimachi, K.; Shimizu, H. PCT Int. Appl. WO 2005070875, 2005.).
The strengths of these chiral phosphoric acids is governed by:
Akiyama Chem Rev 2007
Terada ChemComm 2008
Organocatalysts
First report: Taylor, M. S.; Jacobsen, E. N. J. Am. Chem. Soc. 2004, 126, 10558-10559
In 2009 Jacobsen reported asymmetric Pictet-Spengler reactions cocatalyzed by a chiral thiourea and benzoic acid. A number of optically active tetrahydro-β-carbolines were obtained in high ee.

The catalytic cycle for this was proposed where imine protonation is induced by a thiourea catalyst via H-bonding to the conjugate base of a weak Bronsted acid additive. The highly reactive protioiminium ion then cyclizes and aromatizes to generate the desired product and Bronsted acid cocatalyst. Examples also show that this thiourea catalyst promotes highly enantioselective Pictet-Spengler reactions on electronically and structurally diverse substrates.

Jacobsen published further work continuing with his cocatalyzed thiourea/benzoic acid Iso-Pictet-Spengler reactions in 2011. Here he focused on the synthesis of optically pure tetrahydro-γ-carbolines. He reports a straightforward procedure for upgrading the ee of the tetrahydro-γ-carbolines products by Boc protecting the free amine. This simple step elevates the ee to greater than 99% in nearly all the examples shown and by simple crystallization or trituration. Furthermore, the use of ketone substrates was also demonstrated and shown to proceed to similar yields and ee’s

To Do:
Iso-Pictet-Spengler (C3 of indole) Jacobsen 2011
- MNR Done
Thiourea plus a proton: Jacobsen 2009 - MNR Done
Mechanism
Interesting --> Cook et al, "Study of the Cis to Trans Isomerization of 1-Phenyl-2,3-disubstituted Tetrahydro-β-carbolines at C(1). Evidence for the Carbocation-Mediated Mechanism" DOI: 10.1021/jo8028168 - Proposes mechanism for the racemisation via retro Pictet-Spengler of enantioenriched tetrahydro-β-carbolines synthesised from tryptamines and aldehydes.
Miscellaneous Other Systems/Ones not yet used for PS
Enzymatic examples:
Norcoclaurine synthase Tanner 2007
Strictosidine Synthase [10.1021/ja077190z Stoeckigt 2008]
Conclusions, and what's needed in this field
References
Papers included in the review should be listed here when the description of the science is complete. The papers may be found in full at the Mendeley page)
- Catalytic Asymmetric Pictet-Spengler Reaction, J. Seayad, A. M. Seayad and B. List, J. Am. Chem. Soc. 2006, 128, 1086-1087. Paper
