|
|
| Line 4: |
Line 4: |
| <h1><center>Research</center></h1> | | <h1><center>Research</center></h1> |
|
| |
|
| == Amorphous Systems == | | == Solubility Enhancement == |
|
| |
|
| [[image:growth.png|thumb|500px|left]] | | [[image:growth.png|thumb|500px|left]] |
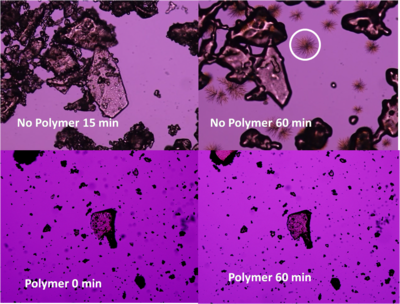
| The amorphous form can be used as a means to enhance the aqueous solubility of very poorly water soluble drugs. However, amorphous solids frequently crystallize with time, negating any solubility advantages. We are interested in understanding why some drugs crystallize more easily than others. Also, it is well known that crystallization can be delayed or inhibited by adding polymers. The mechanism(s) of crystallization inhibition by polymers are not well understood and are being intensively investigated in the Taylor lab. We are currently comparing the inhibitory ability of various common pharmaceutical polymers in the presence and absence of moisture and as a function of temperature. In addition, we are examining the utility of novel cellulose derivatives for preparing amorphous solid dispersions with controlled release profiles, in collaboration with the lab of Professor [http://vtwood.forprod.vt.edu/contactlists/facultydetail.asp?facultyID=52 Kevin Edgar.] Recently, we have discovered that moisture can induce amorphous-amorphous phase separation in some drug polymer mixtures and we are probing the underlying intermolecular interactions and thermodynamics of these systems. We are also studying drug-polymer miscibility and investigating what factors govern if a particular drug and polymer mix. In order to address this question, it is necessary to implement high resolution analytical techniques to assess the miscibility of the systems. Techniques that are currently being evaluated include FT-Infrared Spectroscopy, X-Ray Powder Diffraction, Differential Scanning Calorimetry and various microscopic techniques.
| | Many important substances including new drugs, agricultural chemicals and nutraceuticals are often very insoluble in water. This can make them very hard to formulate and deliver and may even prevent new drugs from being clinically efficacious. Several strategies have emerged to improve aqueous solubility and drug delivery including salt formation, amorphous solid dispersions, cocrystals, complexation, lipid formulations and combinations thereof. The Taylor group is actively involved in research topics pertinent to solubility enhancement and how the different enhancement methods compare with one another. The group has considerable expertise with amorphous formulations, and amorphous solid dispersions are of particular interest. Research in this area encompasses all aspects of the design, production and characterization of amorphous solid dispersions as summarized in the Figure. |
| Once a solid amorphous dispersion has been successfully prepared, it is necessary to understand how enhanced solution concentrations are generated. We are therefore currently developing methods to study the extent and longevity of the supersaturation generated by dissolving amorphous solids and the influence of polymers on the solution levels attained.
| |
|
| |
|
| == Dissolution of Amorphous Solid Dispersions == | | == Dissolution of Amorphous Solid Dispersions == |