Swartz:Research/VLPs: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 1: | Line 1: | ||
{{Template:Swartz}} | {{Template:Swartz}} | ||
The Virus-Like Particles team in the [[Swartz| Swartz Lab]] focuses on developing Biotechnology-based solutions using VLPs. Early work using the cell free protein synthesis platform enabled the site-specific incorporation of non-natural amino acids into our VLPs that allows for the direct bioconjugation of functional molecules to the surface using "click" chemistry. In addition, recent work has allowed us to stablize the VLP with additional disulfide bonds as well as alter the surface charges to allow conjugation of wider variety of ligands. Current work is being done to modify the inner surface of the VLP to control loading of various therapeutic or diagnostic cargoes. | |||
We are currently using our virus-like particle platform to address a range of human health problems such as circulating tumor cell enumeration, HIV and lymphoma vaccine design, cell-specific delivery of therapeutics for various diseases, and improved MRI of prostate cancer. | |||
The | The VLP team currently consists of Benjamin Ko, Marcus Rohovie, Julie Fogarty, Maya Nagasawa, and Rinchu Mathew. Former members of the group include Brad Bundy, Wei Chan, Yuan Lu, Kedar Petal, and Chris VanLang. | ||
[[Image:VLP2.png]] | |||
'''1) VLPs as vaccine scaffolds (Julie Fogarty)''' | '''1) VLPs as vaccine scaffolds (Julie Fogarty)''' | ||
| Line 13: | Line 15: | ||
'''2) VLPs for use as a drug delivery vehicle, including screening capabilities (Marcus Rohovie, Maya Nagasawa)''' | '''2) VLPs for use as a drug delivery vehicle, including screening capabilities (Marcus Rohovie, Maya Nagasawa)''' | ||
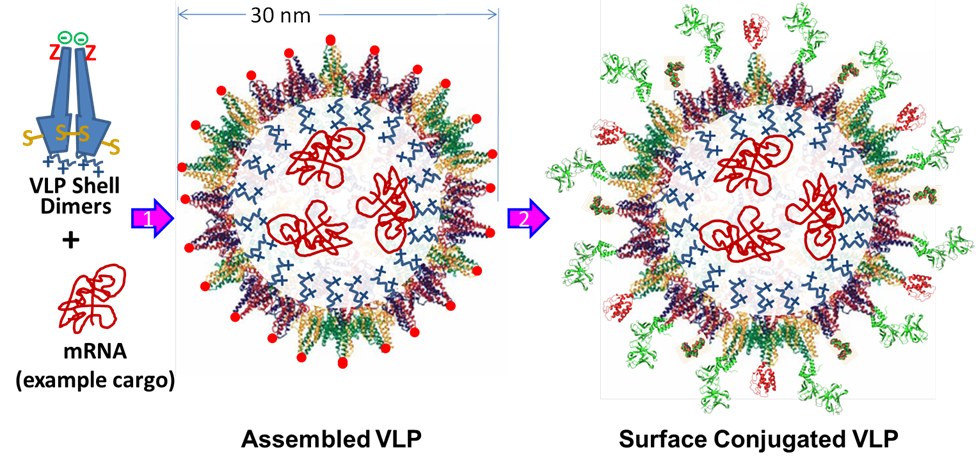
We have engineered a library of VLP mutants with different C-terminal extensions (blue squibble above, shown with positive charges). These mutations alter the functionality of the extension - cationic interactions, anionic interactions, hydrophobic interactions, aromatic interactions, etc. - and will be used to show loading of various therapeutic cargo - chemotherapeutics for prostate cancer, dopamine for Parkinson's Disease, or nucleic acids for treatment of any genetic disorder or disease characterized by a non-functional enzyme. | |||
By using the non-natural amino acids on the surface of the VLPs (red Z above), we can use "click" chemistry to attach various ligands to the surface of the VLP. Most importantly, we can attach antibody fragments that will enable the VLPs to bind to specific cells enabling targeted delivery of the loading therapeutic cargo. Upon binding to the targeted cell, the VLPs will be internalized via receptor-mediated endocytosis. After escaping the endosome (which can be assisted by displaying cell-penetrating peptides on the surface of the VLP as well), the reducing environment of the cytoplasm will reduce the stabilizing disulfide bonds in the VLP and allow it to deliver its cargo. | |||
This method would allow higher doses of the therapeutics to be used since the specific targeting mechanisms of the VLP should reduce off-target side effects. Additionally, there is no modification needed to the drug to allow it to be delivered. Hence, the VLP could also be used as a high-throughput screen for novel drug development. | |||
'''3) VLPs for use in medical imaging/diagnostics (Benjamin Ko, Rinchu Mathew)''' | '''3) VLPs for use in medical imaging/diagnostics (Benjamin Ko, Rinchu Mathew)''' | ||
Our third area of focus relies on targeting of VLPs to specific cells using biomarkers but instead of delivering small molecule drugs, we seek to package an imaging agent. We are currently focused on packaging of FeO nanoparticles for improved contrast and specificity for MRI. We are also developing technology in order to detect and enumerate circulating tumor cells for early detection and diagnosis of cancer cells. | Our third area of focus relies on targeting of VLPs to specific cells using biomarkers but instead of delivering small molecule drugs, we seek to package an imaging agent. We are currently focused on packaging of FeO nanoparticles for improved contrast and specificity for MRI. We are also developing technology in order to detect and enumerate circulating tumor cells for early detection and diagnosis of cancer cells. | ||
Revision as of 11:06, 29 September 2014
| Research | Papers | People | Contact |
The Virus-Like Particles team in the Swartz Lab focuses on developing Biotechnology-based solutions using VLPs. Early work using the cell free protein synthesis platform enabled the site-specific incorporation of non-natural amino acids into our VLPs that allows for the direct bioconjugation of functional molecules to the surface using "click" chemistry. In addition, recent work has allowed us to stablize the VLP with additional disulfide bonds as well as alter the surface charges to allow conjugation of wider variety of ligands. Current work is being done to modify the inner surface of the VLP to control loading of various therapeutic or diagnostic cargoes.
We are currently using our virus-like particle platform to address a range of human health problems such as circulating tumor cell enumeration, HIV and lymphoma vaccine design, cell-specific delivery of therapeutics for various diseases, and improved MRI of prostate cancer.
The VLP team currently consists of Benjamin Ko, Marcus Rohovie, Julie Fogarty, Maya Nagasawa, and Rinchu Mathew. Former members of the group include Brad Bundy, Wei Chan, Yuan Lu, Kedar Petal, and Chris VanLang.
1) VLPs as vaccine scaffolds (Julie Fogarty)
We are currently developing VLPs for the vaccination against B cell lymphoma and HIV. Using the VLP we can conjugate specific immuno stimulatory agents on the surface simultaneously with specific disease carrying epitopes to trigger the native immune response.
2) VLPs for use as a drug delivery vehicle, including screening capabilities (Marcus Rohovie, Maya Nagasawa)
We have engineered a library of VLP mutants with different C-terminal extensions (blue squibble above, shown with positive charges). These mutations alter the functionality of the extension - cationic interactions, anionic interactions, hydrophobic interactions, aromatic interactions, etc. - and will be used to show loading of various therapeutic cargo - chemotherapeutics for prostate cancer, dopamine for Parkinson's Disease, or nucleic acids for treatment of any genetic disorder or disease characterized by a non-functional enzyme.
By using the non-natural amino acids on the surface of the VLPs (red Z above), we can use "click" chemistry to attach various ligands to the surface of the VLP. Most importantly, we can attach antibody fragments that will enable the VLPs to bind to specific cells enabling targeted delivery of the loading therapeutic cargo. Upon binding to the targeted cell, the VLPs will be internalized via receptor-mediated endocytosis. After escaping the endosome (which can be assisted by displaying cell-penetrating peptides on the surface of the VLP as well), the reducing environment of the cytoplasm will reduce the stabilizing disulfide bonds in the VLP and allow it to deliver its cargo.
This method would allow higher doses of the therapeutics to be used since the specific targeting mechanisms of the VLP should reduce off-target side effects. Additionally, there is no modification needed to the drug to allow it to be delivered. Hence, the VLP could also be used as a high-throughput screen for novel drug development.
3) VLPs for use in medical imaging/diagnostics (Benjamin Ko, Rinchu Mathew)
Our third area of focus relies on targeting of VLPs to specific cells using biomarkers but instead of delivering small molecule drugs, we seek to package an imaging agent. We are currently focused on packaging of FeO nanoparticles for improved contrast and specificity for MRI. We are also developing technology in order to detect and enumerate circulating tumor cells for early detection and diagnosis of cancer cells.