Stanford/BIOE44:Lab essentials: Difference between revisions
| Line 226: | Line 226: | ||
==For Next Time== | ==For Next Time== | ||
'''"For Next Time" are short exercises that you can complete at the lab computers (if you finish all your other work before the end of lab) or at home. | '''"For Next Time" are short exercises that you can complete at the lab computers (if you finish all your other work before the end of lab) or at home. Much of the daily lab quiz questions will reflect and assess your understanding of the "For Next Time" materials.''' | ||
Complete the Stanford Biosafety Training online prior to the 1 April 2010 class. To do this go to [https://axess.stanford.edu/ Stanford Axess], log in, click the "STARS (Training)" link, search the catalog for "EHS-1500", enroll and complete the course. | Complete the Stanford Biosafety Training online prior to the 1 April 2010 class. To do this go to [https://axess.stanford.edu/ Stanford Axess], log in, click the "STARS (Training)" link, search the catalog for "EHS-1500", enroll and complete the course. | ||
Once you successfully complete EHS-1500 you should receive an email. Please forward this email to Drew Endy. | Once you successfully complete EHS-1500 you should receive an email. Please forward this email to Drew Endy. | ||
Revision as of 09:02, 30 March 2010
adapted from MIT 20.109
There are six stations for you and your lab partner to visit on your lab tour today. Some will be guided tours with a TA or faculty there to help you and others are self-guided, leaving you and your partner to try things on your own. Your visit to each station will last 15 minutes. It doesn’t matter which station you visit first but you must visit them all before you leave today. If you finish a station before the group ahead of you is finished, please go to the computer cluster and create an open wetware account for yourself. Once your account is approved, at home, add yourself to the people page of our course website.
Station 1: Introduction to pipetting
(Guided: Kosh)
Someone will show you how to use your pipetmen and then you will use them to dilute a blue dye (0.01% Xylene Cyanol).
- If you have never used pipetmen then you should practice by pipeting 800, 80 and 8 μl of the 0.01% XC stock into eppendorf tubes. XC is not hazardous but it will stain your clothes. Pipet each volume three times and visually inspect how well the volumes match.
- Using your P20, measure 10, 15 and 20 μl of the 0.01% XC stock solution into the bottom of three cuvettes. Using your P1000, add water to bring the final volume to 1 ml (=1000 μl).
- Using your P200, measure 20, 50 and 100 μl of the 0.01% XC stock solution into the bottom of three more cuvettes. Using your P1000, add water to bring the final volume to 1 ml.
- Using your P1000, measure 100, 200, and 400 μl of 0.01% XC solution into the bottom of three more cuvettes. Add water to bring the final volume to 1 ml.
- With a gloved hand or with a piece of parafilm over the lip of the cuvette, invert each cuvette several times to thoroughly mix the contents.
- Visually compare your dilutions to the reference ones. If time permits, you will read the absorbance of your dilutions in the spectrophotometer so do not throw them away.
Station 2: Introduction to our spectrophotometer
(Self-Guided)
Color is created when a white light strikes a molecule that then reflects light of a certain wavelength and absorbs all the others. A spectrophotometer is an instrument that measures the amount of light absorbed by a sample. It does this by shining light of a particular wavelength into a sample and measuring how much light comes all the way through. Samples are held in cuvettes between the light source and the detector.

Here are two important things to remember about spectrophotometers. First, different compounds absorb different wavelengths of light. Red pigments absorb blue light (light of ~300 nm wavelengths) and blue pigments absorb red light (light of ~600 nm wavelengths). Therefore all spectrophotometers have ways of adjusting the wavelength of light shining into the sample. The second important point is that the amount of light absorbed by a sample is directly proportional the concentration of that sample. This is a very useful relationship, making the spectrophotometer a valuable research tool.
Part 1: Using the Spectrophotometer
In this assay you will calibrate your pipets by measuring the absorbance of the XC dilutions you made. Beer’s Law, which relates absorbance to concentration, will be derived as part of experimental module 2. Here, you’ll see that the graph of absorbance versus volume of 0.01% XC is a straight line….or at least it should be!
- Using your P1000, measure 1 ml of water into a plastic cuvette. This cuvette will serve as your blank for the spectrophotometer.
- Confirm that the machine is set to read absorbances at 600 nm.
- Put your blank into the spectrophotometer at position 1, which is furthest back in the instrument. Be sure the window of the cuvette and not the frosty sides are in the light beam that travels from left to right.
- Close the door of the spectrophotometer. Click “blank” (lower left of the screen). A “reading blank” message should appear. When the message is gone, then the blank is set.
- Replace the blank with your first sample. Close the door of the spectrophotometer. Click “read samples” (upper left of the screen). Write down this value.
- Repeat with all your samples.
- Remove your last sample. Close the door of the spectrophotometer.
- The XC dilutions can be washed down the sink and the cuvettes can be discarded in the sharps bin.
Part 2: DNA and Nano-Drop
(Self-guided, but please ask Kosh if you have ANY questions).
The NaneDrop is a spectophotometer often used to measure the purity of a solution containing biomolecules such as DNA and proteins. It is convenient because it only requires a small amount of liquid (1-2 microliters) for each sampling. For instance, you will use it often as a quality assurance step, to ensure your solutions contain sufficient DNA, after extracting plasmids from a culture. The NanoDrop tells you what wavelengths of light are being strongly absorbed by your solution. Since DNA and amino acids absorb at different wavelengths, the NanoDrop can be useful for measuring the purity of a solution.
To use the NanoDrop 2000:
- Ensure the monitor and computer power are switched to on.
- Click on the shortcut to the program NanoDrop 2000 on the desktop
- Select the button Nucleic Acids
- Ensure that the device arm is down and click OK to run a routine wavelength verification.
- You will need to run a BLANK. Here the machine will set its zero-point, akin to when one sets a zero point on a scale.
- Gently spray a small amount of distilled water on the sampling port. Gently wipe off the liquid with a Kim wipe.
- With a p2, carefully place 1.5 micro-liters of MQ water on top of the measuring port. The measuring port looks like a mini-nozzle where you might stick a needle into a soccer ball.
- Gently close the device arm. And hit the "Blank" icon in the upper left hand corner of the screen. You will here a click. Wait until the machine has taken a reading. The reading should be zero.
- Again using a Kimwipe, wipe off any residual liquid.
- Apply 1.5 micro-liters of the liquid sample labeled "Mystery DNA" atop to the sampling port, close the device arm and hit the measure icon. (This Icon looks like the play button on iTunes). You should hit Cancel when prompted to save a workbook.
- You will need to record three important pieces of data in your lab notebook. WHEN YOU ARE FINISHED CLOSE THE PROGRAM SO THAT THE NANO DROP IS RESET FOR THE NEXT GROUP.
DNA Concentration ___ng/µl _____260/280 ratio* _____260/230 ratio**
- The 260/280 ratio provides an estimate of purity of a solution with nucleic acids. A target 260/280 ratio is 1.8. A higher ratio suggests that your solution contains proteins as well as DNA.
- The 260/230 ratio is a second measure of nucleic acid purity. A ratio less than 2.0-2.2, suggests the presence of other contaminants, often organic solvents.
Part 3: Considering your data
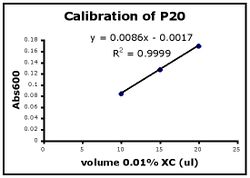
- Use Excel to prepare a graph of absorbance versus volume of 0.01% XC. Some sample graphs are reproduced below and you should generate similar ones with your data. Be sure to include a trendline, displaying its equation as well as the r-squared value on the graph. The r-squared value reflects how well the data points fit the equation. A perfect fit will give an r-squared value of 1. If you are uncertain how to make such a graph using Excel, be sure to ask for help.
- If the pipets were well calibrated and the measurements were done carefully, then the points should fall close to a straight line, and the r-squared will be close to 1. If one point seems way off, you can repeat the three measurements for that pipetman. If the second set of data does not look linear, we can clean the inner workings of your pipetman before you try the assay again.
- There should also be good agreement between the 20 ul measurements made with the P20 and the P200 as well as the 100 ul measurements made with the P200 and P1000. Is there?


Station 3: Introduction to lab math
(Self-Guided)
The information and exercises provided here are intended to refresh your memory of these concepts. If they are entirely new to you or if you are struggling with the practice problems, please ask for extra help. It is absolutely essential that you are comfortable with the information presented here.
Part 1: Metric system
This is the numerical language of science. Base units that you will most often use in this class are meters, grams, liters, and moles. These units will be appended with prefixes to modify the unit by a power of ten.
103 = 1000 = 1000/1 = 103/1 kilo (k-) 100 = 1 = 1/1 = 100/1 base unit (-g, -l, -mole…) 10-3 = 0.001 = 1/1000 = 1/103 milli (m-) 10-6 = 0.000001 = 1/1000000 = 1/106 micro (μ-)
Practice problems:
- The distance between two cells in 800 μm. How many mm is that?
- The amount of sorbitol you want to weigh is 1.9 g. How many mg is that?
- The volume you want to measure is 100 ml. How many liters is that?
- Your reaction generates 0.1 μmoles of product. How many mmoles is that?
Scientific notation expresses numbers so there is one digit to the left of the decimal point and that number is multiplied by a power of ten. 2334 becomes 2.334 x 103 and 0.0041 becomes 4.1 x 10-3. Computations are easier with numbers in scientific notation and some numbers that are easier to write (602,214,199,000,000,000,000,000 versus 6.02 x 1023).
Practice problems: Convert the following to scientific notation
- 1000
- 2
- 0.0023
- 0.000000467
The metric system and scientific notation go hand in hand, making unit conversions straightforward. For example 100 μl can be converted to ml by writing the starting volume in scientific notation (1.00 x 102 μl) and multiplying by the power of ten that separates the units (1 ml = 1 x 103 μl). Set up every equation so the units will cancel properly when you multiply through.
Practice problems: Be sure you can express your answers in scientific notation.
- How many ml is 100 μl?
- How many mg is .023 g?
- How many mmoles is 250 μmoles?
Part 2: Concentrations
Molarity (moles/liter) is a common expression of concentration. When making a solution of a particular molarity, you need to know three things: the desired molarity, the desired volume and the formula weight of the compound to be dissolved. The best place to find the formula weight (grams/mole) is on the chemical’s bottle. Calculations are performed by setting up an equation so that the units cancel, leaving grams in the numerator and volume in the denominator.
Another common expression of concentration is percent. Percent solutions are always based on 100 ml. For powdered substances, percent solutions reflect the weight in a 100 ml volume (“w/v”). For example a 10% solution of NaCl is 10 grams in 100 ml of water. In fact a 10% solution of any powdery substance is 10 grams in 100 ml. For liquids, percent solutions reflect the volume in a 100 ml final volume (“v/v”). For example a 70% ethanol solution is 70 ml of 100% ethanol and 30 ml of water. Remembering that 1 ml of water weighs 1 gram may help you remember the w/v and v/v expressions.
Practice problems:
- You want to make 100 ml of a 0.5M sorbitol solution. The formula weight of the substance you want to dissolve is 182. How many grams will you measure?
- You want to make 10 ml of a 0.01% (w/v) solution of XC. How many grams will you dissolve?
- How would you make 100 ml of a solution that is 5% (v/v) acetic acid and 5% methanol?
Part 3: Dilutions
Many solutions are made by diluting concentrated stock solutions. Dilution factors of 1:2, 1:5, 1:10 and 1:100 are common. These dilutions are made by diluting one “part” stock with 1, 4, 9 or 99 “parts” water. For example, you could make 100 ml of a 0.5M sorbitol solution by mixing 10 ml of a 5M stock solution with 90 ml of water. This is a 1:10 dilution of the stock. The dilution factor can be converted to a fraction to determine the solution’s final concentration (5M x 1/10 = 0.5M).
When the dilution factor is less obvious, the formula C1V1 = C2V2 can be used, where C1 is the starting concentration of the stock solution, C2 is the desired concentration, V1 is the volume of stock you’ll need (usually this is your unknown) and V2 is the final volume you want to make. For example, to make 1000 ml of a 0.2M Tris from a 1.5M stock you would multiply 1.5M (V1) = 0.2M (1000) to find that you will need 133 ml of the stock. To determine how much water to add you would subtract V2 – V1, in this case 1000 ml –133 ml = 867 ml of water.
When solutions must be diluted several orders of magnitude, then serial dilutions are made. The concentrated stock is progressively diluted, for example using a 1:100 dilution as the new “stock” in another 1:100 dilution. Such a serial dilution produces a solution that is 10,000 times less concentrated than the starting material. One benefit to serial dilutions is that small volumes of each dilution can be made accurately. A drawback is that any pipetting or calculation error is propagated through every dilution.
Practice Problems
- How would you make 50 ml of a 1:5 dilution?
- Give the volume of stock and the volume of water necessary to make 50 ml of a 0.25 M solution starting with a 2M solution.
- A concentrated culture of bacteria has approximately 1 x 108 cells/ml. What is the concentration of bacteria after it has been diluted 1:100? What is the concentration of bacteria if a 1:2 dilution was made of the 1:100?
Station 4: Introduction to making solutions
(Self-Guided)
Today you will make 100 ml of a 0.5M urea solution and measure its pH. Making solutions is a fundamental part of being in lab and the success of your experiments is absolutely dependent on doing it correctly and consistently. If you are unclear about any of the following instructions, be sure to ask for help. (NOTE: UREA IS A MILD SKIN AND RESPIRATORY IRRITANT. YOU SHOULD WEAR GLOVES AND EYE PROTECTION WHILE MEASURING IT OUT ON BALANCE)
Part 1: At the Balance
- Put on gloves to weigh out solids. This protects you from the chemicals and the chemicals from getting contaminated with anything foreign on your hands. Urea is not a dangerous chemical.
- Zero the balance with a medium size weigh boat on it. Weigh boats are kept in the drawer under the balance. The marked -> O/T <- will zero (“tare”) the balance and the display should read 0.00 after taring.
- Use a spatula to measure 3 grams of urea. To measure this, open the balance doors and hold the spatula and chemical over the weigh boat. Begin by adding only a small amount of the powder to the weigh boat. Once you determine how much that weighs, you can add correspondingly more. If you have weighed out too much, you can put some back as long as you have used a clean spatula and a clean weigh boat.
- Remove the weigh boat with your urea from the balance, gently bend the ends together and pour the contents into a beaker. Tap the back of the weigh boat to loosen any powder that is stuck. The weigh boat can be discarded in the trash since urea is not dangerous.
- Clean the balance with a kimwipe. Clean the area around the balance with a wet paper towel.
Part 2: Measuring Liquids and Mixing
- Measure approximately 80 ml of ultrapure water into a 100 ml graduated cylinder. Read the volume in the cylinder by bringing it to eye level to see where the meniscus reaches. Add the water to the beaker with your urea.
- Gently drop in a magnetic stir bar with a diameter approximately 1/2 that of the beaker. Magnetic stir bars are kept in the drawer below the stir plates.
- Put the beaker on the stir plate and turn the stirrer on slowly. The stir bar should spin fast enough to form a vortex in the center of the beaker. You do not want the stir bar to bump around in the beaker since this can break the beaker. If the stir bar is stirring unevenly, then turn off the stir plate, allow the magnetic stir bar to stop, and then start it again.
- Stir until all the powder is dissolved.
- Pour the solution back into your graduated cylinder.
- Carefully add ultrapure water up to 100 ml. It may be helpful to dispense water into another container (like a graduated cylinder) then slowly pour it into your urea solution until you reach 100mL.
Part 3: Measuring pH
- Since you will measure the pH of your solution you should pour it back into your beaker and put it on a stir plate. Start the stir bar gently spinning. If you did not have to measure its pH you would move it to a bottle for storage.
- Remove the pH electrode from the storage solution and rinse it over the waste beaker using distilled water from the wash bottle. Be careful to leave the top cap of storage solution on the electrode. You should move it up the column of the electrode so that it does not make contact you sample liquid. A Kimwipe can be used to gently dry the electrode.
- Place the electrode 1/2 way into the 15 ml conical tube with calibration buffer pH 7.0. Wait for the reading to stabilize and note how close it is to pH 7. A display should read "Stable," at which point you should record this proton concentration.
- Rinse the electrode then gently dry it with a Kimwipe. Place it in your urea solution. Hold the electrode at the edge of the beaker and be careful not to let the stir bar hit (and break!) the electrode. Read the pH of your solution and let one of the teaching faculty know what you have found.
- Rinse and dry the electrode then return it to the electrode storage solution.
- Pour the urea solution (but not the stir bar!) down the sink. Rinse the beaker and graduated cylinder with tap water and return it to the balance area. Rinse and Dry your spatula and return it to the drawer.
Station 5: Sterile Technique
(Guided: Isis) Adapted from "At the Bench." Kathy Barker 2005.
As many as 1,800 bacterial phylotypes have been detected in urban air (Brodie et al. 2007), and the average human hand harbors more than 150 unique bacterial phylotypes (Fierer et al. 2008). Sterile technique should be used to minimize the exposure of your experimental material to contamination by foreign microorganisms and DNA. Put bluntly, the air above your bottles and cultures contains dust, spores, and potential contaminants. The same is true for the surface of your lab bench. You want to minimize the amount of time sterile or experimental media is exposed to the air, and when they are exposed, ensure that air currents blow potential contaminants away from your working area. Your Bunsen burner will be a useful tool for this purpose.
Some guidelines to follow (you should follow each step at your bench):
- Wipe down your bench. Use an antiseptic and clean paper towels. A 70% ethanol solution works well in most cases. A bleach solution is also often used before working with DNA. Keep a bottle of antiseptic agent nearby.
- Arrange your working area to minimize the distance between tools. Place the Bunsen burner in the center of your workspace. Place the tools you will use with your right hand to the right of this open area. Similarly, place the tools you will use with your left hand to the left of this open workspace. Remember that when you open a container, you should keep it beneath, and close to, the open flame (within 6 inches is close enough). Keep the distance between containers to a minimum.
- Prepare. Make sure you have everything you need before opening any of your experimental materials.
- Use the 45º angle technique. That is, when you open bottles, hold them at a 45º angle. This will minimize the chance that contaminating organisms fall into your liquid media.
- Mind the cap. When you take a cap off a bottle, place it face down, or sideways, on a sterile surface. Do not leave bottles open for any longer than is necessary. Immediately cap all solutions when you are finished with them.
- Pipette don't pour. Pouring creates aerosols and creates opportunities for contamination. Whenever possible, transfer liquids by pipette.
- Watch your pipette tip. Be careful not to touch the bottom section of your pipette as it will enter the solution. Don’t waive your micropipette. When using a micro-pipette, make sure not to touch the pipette tip to anything before it enters your solution. Waving your pipette through the air defeats your efforts at maintaining a sterile working area. Practice removing the pipette tip from the tip box, and directly moving it a short distance to your solution.
- Flaming. you will receive more instructions regarding flaming your bottles and instruments in future lab sessions. Remember NOT to flame any plastic tools.
- See Kathy Barker's "At the Bench" pages 190-196 for more practical tips about moving efficiently to minimize contamination.
Station 6: Lab Safety
Taking responsibility for your own safety is important in every laboratory. In this lab space, in particular, you should exercise good judgment. The Civil and Environmental Engineering department used this lab for many years as a research facility. As such, harmful reagents were likely used in the fume hoods.
Here are some guidelines to help maintain your safety as well as that of your bench-mates.
Basics
- No sandals or open-toed shoes in the lab.
- Never bring food or drink into the lab.
- No pipetting by mouth. (Believe it or not, people once did this. YOU SHOULD NOT).
- Always wash your hands with SOAP before leaving.
- Do not use fume hoods unless instructed to do so. Stay within areas of the lab designated for your use.
- Avoid touching doorknobs or computer clusters with gloved hands. (This protects you and others when exiting the lab).
- Never take pipettes, reagents, cultures, or any other laboratory materials out of the lab.
- Get in the habit of labeling liquids you are using. (Label the contents of any solution and include the date it was prepared).
- If glass breaks, do not pick it up by hand. First, tell a TA. Then use a broom and dust-pan to pick it up.
- Always report an accident or spill immediately.
Flames
- Tell your partner when you turn on your Bunsen burner.
- Be careful when wearing nitrile or latex gloves next to an open flame. If they melt, gloves can cause severe burns.
Gel Station Electrophoresis often employs ethidium bromide (EtBr) to fluorescently tag DNA. EtBr is a mutagen.
- You should always wear gloves and eye protection when working with EtBr.
- When you are done at the Gel station, remove and throw away gloves before returning to your bench.
- Do not use your own pipettes and beakers at the gel station. Tools will be provided.
UV Light Boxes
- Limit exposure and wear proper protective eyewear and long-sleeved clothes when using UV boxes
Before you leave for the next module
- Locate at least two eye-wash stations and showers in the lab.
- Locate a phone where you would be able to call 9-911 in an emergency
For Next Time
"For Next Time" are short exercises that you can complete at the lab computers (if you finish all your other work before the end of lab) or at home. Much of the daily lab quiz questions will reflect and assess your understanding of the "For Next Time" materials.
Complete the Stanford Biosafety Training online prior to the 1 April 2010 class. To do this go to Stanford Axess, log in, click the "STARS (Training)" link, search the catalog for "EHS-1500", enroll and complete the course.
Once you successfully complete EHS-1500 you should receive an email. Please forward this email to Drew Endy.
