SBB12Ntbk-Annie Joseph: Difference between revisions
Annie Joseph (talk | contribs) |
Annie Joseph (talk | contribs) |
||
| Line 161: | Line 161: | ||
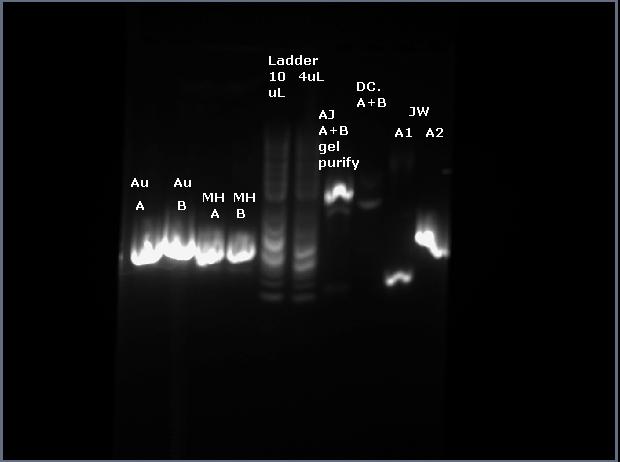
The analysis shows no bands on the gel lane: | The analysis shows no bands on the gel lane: | ||
[[image:2012_03_01_gel2_ssb2012spring.jpg]] | |||
This is strange because there was no DNA present, which I think indicates a failure in the miniprep step. However, the transformation step was rushed, so I will go back and do another ligation/transformation. | This is strange because there was no DNA present, which I think indicates a failure in the miniprep step. However, the transformation step was rushed, so I will go back and do another ligation/transformation. | ||
Revision as of 17:47, 1 March 2012
Annie Joseph 16:23, 13 February 2012 (EST)
We did initial PCR/PCA today!
For part sbb1216:
Obtained and made a 10 micro molar solution of oligos ca998/osbb1333R and osbb1216F/g00101 for PCR.
Set up two solutions for PCR:
This one I call A in construction file:
24uL ddH2O
3.3uL 10x Expand Buffer "2"
3.3uL dNTPs (2mM in each)
1uL ca998, 10uM
1uL osbb1333R, 10uM
0.5uL Expand polymerase "1"
0.5uL pBca9145-jtk2768
And...
This one is B:
24uL ddH2O
3.3uL 10x Expand Buffer "2"
3.3uL dNTPs (2mM in each)
1uL osbb1216F, 10uM
1uL g00101, 10uM
0.5uL Expand polymerase "1"
0.5uL pBjk2741-jtk3346
For part sbb1205
I set up a solution based on the PCA protocol on open wet ware.
38 uL ddH2O
5 ul 10x expand buffer
5 ul 2mM dNTPs
1 ul oligo mixture (100uM total, mixture of oligos after combination of 100uM stocks)
0.75 ul Expand polymerase
Notes:
n/a
Annie Joseph 12:30, 17 February 2012 (EST)
Ran two lanes on agarose gel for gel purification of products A and B from last time. Extracted the product A from the gel. The lane
for B was very faint so I have decided to redo the gel one more time before considering whether it was a mistake in an earlier step :(
Created the PCA solution for creation of pca2.
For next time: I will rerun the gels (PCR A&B with loading buffer), extract Create the digests for the part and template and use thermocycler (time for ligation ?)
Possibly with time remaining, I will set up the pcr reaction with A+B
Annie Joseph 12:30, 21 February 2012 (EST)
For the sbb1216 part, I ran the two PCR products from last week on the agarose gel for gel purification:
PCR ca998/osbb1333R on pBca9145-jtk2768 (A)
PCR osbb1216F/g00101 on pBjk2741-jtk3346 (B)
Obtained alright results, can be seen on the gel two lanes 8 and 9. The bands (brightest ones) look to be about the right size.
Cut out the bands and did the rest of the gel purification for both A and B.
For the Leucine Zipper part (sbb1205):
I had the pca2 product from last week, which I ran on gel for analysis (lane 4 ?) looks alright.
Did a cleanup of the pca2 product (did the protocol for regular zymo cleanup which I think might have been better to use the small frag
cleanup given the size of the pca2) and proceeded to do the digest of pca2 with NheI/BamHI following the protocol for EcoRI/BamHI
Digest of Wobble Products for the size considerations. Kept the digest on 37 degrees on the thermocycler for ~1 hour. Finally did a
zymo small fragment cleanup this time.
Annie Joseph 12:30, 23 February 2012 (EST)
sick :(
Annie Joseph 12:30, 24 February 2012 (EST)
Set up the A+B PCR with parts ca998/g00101 for my sbb1216 part
Same PCR protocol as before but there are two templates A and B from last tuesday that is supposed to anneal at their complementary
region as part of the Soeing PCR process
Also attempted a transformation but underestimated the time requirement
Ligated the PCA2 digest with NheI/BamHI with the Bca1834 vector digest NheI/BamHI.
Allowed the Ligate product to incubate for 30 minutes on benchtop.
Went through the protocol for transformation with 200 micro liters of cells.
- Last step modification: (not sure how it will affect the cells ability to have grown) only incubated the cells with ligate for around
35 minutes versus 1 hour after heat shock.
Annie Joseph 12:30, 28 February 2012 (EST)
Zach picked the colonies over the weekend and had them growing in tubes when we arrived today. He picked 4 colonies (white, the red
colonies indicates failure of transformation and there were some red colonies). Only one of my colonies out of 4 grew. I did miniprep
procedure to isolate the plasmid DNA from this colony of cells using the protocol listed in open wet ware. The extracted DNA has
been saved in a tube (eluted with water to a total volume of 50 micro liter). Now I need to digest and run an analytical gel before
deciding whether or not to sequence.
I also picked three more colonies from the plate to grow overnight to see if I have any success growing more, see if more of them
transformed.
For the other part I am working on (sbb1216) I got back the pcr product for the A+B pcr reaction with end oligos. Ran it on gel,
results will determine whether I can digest, ligate, etc.
Annie Joseph 12:30, 1 March 2012 (EST)
None of the picked colonies grew :(
So I have only one colony to work with which was miniprepped last time. Today I am running a digest of it to do an analytical gel analysis.
The analysis shows no bands on the gel lane:
This is strange because there was no DNA present, which I think indicates a failure in the miniprep step. However, the transformation step was rushed, so I will go back and do another ligation/transformation.
Also have the A+B on gel to gel purify and set up a digest of A+B. The A+B band is too big (~3000 bp when it should be ~1500):