SBB10Ntbk-AmyKristofferson: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
[http://openwetware.org/wiki/SynBioBootcamp:Notebook/SynBioBootcamp10 BioE140L 2010 Homepage]<br> | [http://openwetware.org/wiki/SynBioBootcamp:Notebook/SynBioBootcamp10 BioE140L 2010 Homepage]<br> | ||
[http://openwetware.org/wiki/Template:SBB10Project-19244 My Project Page] | [http://openwetware.org/wiki/Template:SBB10Project-19244 My Project Page] | ||
==3/1/10: New plan of action | |||
Redo SOEing PCR, using a different temperature? 2K45? Use DMSO? (See PCR in Practice) | |||
==2/24/10: SOEing PCR and Analytical Gel== | ==2/24/10: SOEing PCR and Analytical Gel== | ||
===Wetlab Notes=== | ===Wetlab Notes=== | ||
Revision as of 13:47, 1 March 2010
BioE140L 2010 Homepage
My Project Page
==3/1/10: New plan of action
Redo SOEing PCR, using a different temperature? 2K45? Use DMSO? (See PCR in Practice)
2/24/10: SOEing PCR and Analytical Gel
Wetlab Notes
- PCR gh1000F/ak11R on A+B+C using this protocol and 55 PCR.
- Ran analytical gel to verify that desired PCR product was created. (Protocol: add 3uL PCR product and 7uL of loading buffer)
 My PCR product was labeled sbb22 and was in the fourth lane from the left. The PCR product should have been 619bp, but all the bands that showed up were under 500 bps.
My PCR product was labeled sbb22 and was in the fourth lane from the left. The PCR product should have been 619bp, but all the bands that showed up were under 500 bps.
- Performed a Zymo clean-up to remove dNTPs, Expand Polymerase, and oligos. What's left: the <500bp PCR product, A, B, C. DNA labeled "sbb22, final PCR product, AK" and placed in Box A.
Protocol Update
Next few steps:
Today:
Next time:
- Eco/Bam Digest
- Zymo clean up to remove small fragments and restriction enzymes
- Run a preparative gel to separate digested product from A, B, and C and to ensure product was correctly digested (Protocol: add 6uL PCR and 4uL of loading buffer)
- Cut out digested product and perform a Zymo clean-up
2/22/10: Preparative Gel for PCR product A,B,C
Mixed 6uL of each PCR product with 4uL of loading buffer and ran the gel.

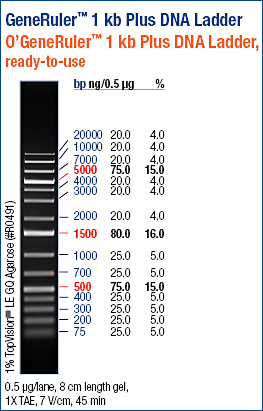
My PCR products A, B, and C were in lanes 2, 3, and 4.
I cut out the bottom bands (the upper band is most likely template DNA), performed a small fragment Zymo Cleanup, and stored the eluted DNA in Box A.
2/17/10: New construction file, SOEing Protocol, PCR of A,B,C off of template
New sbb22 Construction File
Construction of FokI- basic part
PCR gh1000F/gh1001R on BBa_K243001 (261 bp, gp = A)
PCR gh1001F/gh1003R on BBa_K243001 (264 bp, gp = B)
PCR gh1003F/ak11R Registry part BBa_K243001 (141 bp, gp = C)
---------------------------------------------------
PCR gh1000F/ak11R on A+B+C (619bp, EcoRI/BamHI)
Digest pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-sbb22 {<FokI->}
---------------------------------------------------
gh1000F Forward for part <FokI-! ccagtGAATTCatgAGATCTCAGCTGGTTaaatctgaactggaggag
gh1001F Making internal mutation 1+2 cgttggttccccgatcgattatggcgttatcgtggAcacaaaagc
gh1001R Making internal mutation 1+2 cgccataatcgatcggggaaccaacggtataaatggcaccGTctggtttac
gh1003F Making internal mutation 3 ccatatcaccaatTgcaatggggcagtgctgag
gh1003R Making internal mutation 3 ccattgcAattggtgatatgg
ak11R Reverse BamHI for FokI- CTGATggatccaaaattgatctcgccattg
SOEing Protocol for sbb22
- Set up PCR reactions according to your construction file as normal 33uL reactions as described in Cloning by PCR
- For each PCR, load 6uL of PCR product premixed with 4uL of loading buffer in a single well of a 1% agarose gel
- Cut out the bands, put them into a single 1.5mL microcentrifuge tube
- Add 650uL of ADB Buffer
- Proceed with the Zymo Gel Purification procedure
- Elute the DNA in 50uL of water
- Set up your second round of PCR as a normal 33uL reaction using the eluted mixture of fragments as template
Wet Lab notes
Prepared oligos and set up three PCR tubes using this protocol.
2/8/10: Construction Files for my parts
sbb22
Construction of FokI- basic part
PCR ak10F/ak20R Registry part BBa_K243001 (230 bp, gp = A)
PCR ak20F/ak30R Registry part BBa_K243001 (71 bp, gp = B)
PCR ak30F/ak40R Registry part BBa_K243001 (245 bp, gp = C)
PCR ak40F/ak11R Registry part BBa_K243001 (135 bp, gp = D)
---------------------------------------------------
PCR ak10F/ak11R on A+B+C+D (619bp, EcoRI/BamHI)
Digest pBjk2741-Bca1144 (EcoRI/BamHI, 910+2170, L)
Product is pBjk2741-Bak1234 {<FokI->}
---------------------------------------------------
ak10F Forward EcoRI for FokI- CCATAgaattcCAGagatctCAGCTGGTTaaatctgaactggaggagaa
ak20F Base substitution 1 gtaaaccagACggtgccatt
ak20R Base substitution 1 aatggcaccGTctggtttac
ak30F Base substitution 2 cgttatcgtggAcacaaaagcg
ak30R Base substitution 2 cgcttttgtgTccacgataacgc
ak40F Base substitution 3 caccaatTgcaatggggcag
ak40R Base substitution 3 ctgccccattgcAattggtg
ak11R Reverse BamHI for FokI- CTGATggatccaaaattgatctcgccattg
sbb36
Construction of ColE2 Basic Part
Digest pBca9145-jtk2642 (EcoRI/BamHI, 2057+485, S)
Sub into pBca9523- Bca1144 (EcoRI/BamHI, 2472+918, L)
Product is pBca9523-jtk2642 {ColE2 ori med}