SBB10AssayTeam1-LabNotebook: Difference between revisions
Xiao Y. Liu (talk | contribs) |
Wei-Chun Hu (talk | contribs) |
||
| Line 58: | Line 58: | ||
|[[Image:te-5yes.png|thumb|250px|Replicate 3]] | |[[Image:te-5yes.png|thumb|250px|Replicate 3]] | ||
|} | |} | ||
===TECAN Analysis=== | |||
Cells did not show differential growth rates with arabinose induced constructs, as expected. No growth was observed for many wells that are near the edges of the TECAN plate; recommend to not use edge wells in future analyses. | |||
==4/26/2010== | ==4/26/2010== | ||
Revision as of 15:03, 28 April 2010
4/28/2010
 |
 |
ELISA Results
The graph at the right displays a basic breakdown of the absorbance data acquired at the end of the ELISA assay. The four columns indicate:
- No T4L - T4 ligase was not added to these wells, meaning there was nothing for the antibodies to bind in the assay.
- No Myc - The Myc antibody was not added to these wells, meaning there was nothing to bind the T4 ligase to the plate.
- No his-HRP - The his antibody conjugated to HRP was not added to these wells, meaning that the T4 ligase was not attached to a second antibody, and also that no reaction would occur with the HRP to produce a absorbance signal.
- Pos - This is the positive control, so all reagents should have been added at the appropriate parts of the protocol. It should produce the largest absorbance signal if the T4 ligase does contain both myc and his tags and was successfully bound.
From the values of each bar, we can get some information about the effectiveness and background fluorescence of this assay.
Ideally, the 'no his-HRP' control should display baseline absorbance because HRP is the molecule responsible for oxidizing TMB and turn blue which has highest absorbance in 450nm, indicating the existence of T4 Ligase. regardless to whether HRP and TMB exist, there are some chemicals in the solution that display some background absorbance. so this should be treated as a loose baseline to compare the rest of the data to.
The 'no myc' and 'no T4L' controls both produced similar levels of fluorescence to each other. This is consistent with the idea that in both of these controls, there should be no T4L bound to the plate (either because it was not present or had nothing to bind to). Even so, it seems that the his-HRP, even without T4L, was able to bind in some amount to the plate and increase the absorbance signal as compared to the no his-HRP case. It is possible that his would be able to interact with the myc antibody or attach to the plate even with the blocking buffer present. These controls indicate that the absorbance signal must be higher than about 20% to effectively indicate a working ELISA assay.
In fact, the 'pos' control gives a absorbance signal of about 27%, the highest of the four controls. This may indicate that the T4 ligase was effectively bound and measured by the two antibodies. However, the standard deviation from this control is so large that it overlaps with the 'no myc' and 'no T4L' controls. This variation may be due to the differing concentrations of T4 ligase which were added to the wells, or other factors which would require repeats of this experiment.
Suggestions for ELISA
1. Problem: Absorbances remain unchanged/increases with decreasing protein concentration
Possible cause: Protein concentrates saturates available antibody
Solution 1: Reduce protein concentrations below 1/10000X
Solution 2: Perform a second purification step
Solution 3: Increase antibody concentrations available to bind protein (primary detecting (anti-His) or primary capture (anti-Myc))
2. Problem: Absorbance of positive control is not significantly different from negative controls
Possible cause: steric hinderance of dual tag construct.
Solution 1: add a ALA-6 linker between two tags.
Solution 2: put tags on the opposite side of the protein.
second possible cause: background noise.
solution 1: do more replicates.
TECAN data
Legend
- X axis = Cycles (each cycle is approximately 5 minutes)
- Y axis = Absorbance (relative units, at 600 nm)
- A,F (blue) = GP2578
- B,G (red) = GP2599
- C,H (green) = GP2619
Without arabinose induction
 |
 |
 |
With arabinose induction
 |
 |
 |
TECAN Analysis
Cells did not show differential growth rates with arabinose induced constructs, as expected. No growth was observed for many wells that are near the edges of the TECAN plate; recommend to not use edge wells in future analyses.
4/26/2010
Did ELISA. Left lab around 5 pm; last step performed was incubating 1h after adding varying concentrations of T4 ligase to 24 wells.
Protocol (roughly):
- Dilute anti-Myc antibody (1000X) to 1X in TBST, plate 100 uL into well
- Incubate at RT for 1h
- Do three plate washes (TBST with 0.1% Tween)
- Put blocking buffer (2% BSA, in TBST) 100 uL in wells, incubate 1h at RT
- Do three plate washes
- Put 100 uL T4 Ligase at various concentrations (diluted in blocking buffer) (see attached spreadsheet) into wells
- Incubate 1h at 37C
- Do three washes
- Put anti-His antibody conjugated to HRP (10000X) 100uL, dilute to 1X in blocking buffer, 1h at 37C
- Do three washes
- Put detection 3,3’,5,5’-Tetramethylbenzidine (TMB) solution 100uL
- Incubate at 37C for 15 min
- Detect with spectrometer at 450 nm
4/22/2010
- Get column, cut tip, tape it to stabilize somewhere high, place waste column beneath the column.
- Pour in lysate + beads
- Collect drips
- Wash with ice cold PBS (20 mL) (N x 0.5 mL) eject PBS against the column wall to wash lysate off the wall.
- Elute with 4-5 times 1 mL volumes of 300 mM imidazole in PBS
- Put eluant into dialysis column, add ice cold PBS to column, then shake in a speed of 5000mph with 4°C for 30 min, then change to fresh PBS, repeat shaking and change media for several times.
- add ligase, purify and run in gel (Mike did this).
4/21/2010
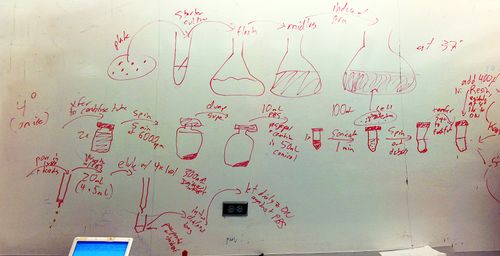
- General protocol for protein purification:
- Pick starter colony
- Grow starter culture
- Put in flask
- Grow to midlog
- Induce arabinose
- Grow to saturation, have protein
- Transfer to centrifuge tube (100 mL) x 2 - stuff here on is on ice (4C)
- Spin 5 min at 6000 rpm
- Dump supernatant, leave pellet
- 10 mL of resuspension buffer (TBS), resuspend and combine in 50 mL conical flask
- Sonicate (probe) - 1 minute (is it a program?). DO pulses.
- Spin to remove debris
- Transfer supernatant to fresh tube (pour). Will be translucent.
- Add 400 uL of Ni resin
- Agitate at 4C 1h to overnight
- Tomorrow
- Get column
- Pour in lysate + beads
- Collect drips
- Wash with PBS (20 mL) (4 x 5 mL)
- Elute with 4-5 1 mL volumes of 300 mM imidazole in PBS
- Put eluant into dialysis bag
- Let dialyze overnight on ice (4°C)
4/19/2010
- analyze TECAN data, maximize TECAN protocol
- TECAN data showed significant edge effects. Recommended to not use outer rows and columns
- I have no clue how to derive the actual growth rate. Tried some logistic equations (a*b)/(a-(b-a)*Exp(-c*t)) and nonlinear regression but my system keeps blowing up, regression looks bad, etc.
- There are a lot of forms of logistic equations you can use. E.x. K / (1 + exp(a + b*x), the differential form dN/dt = r*N(K-N)/K, etc. If anyone has a copy of Mathematica or SPSS, try to play around with it.
- develop ELISA protocol
4/15/2010
- materials: 20% 100X arabinose, LB media and 96 well plate for TECAN
- dilute arabinose to 1X, 50ul LB in each well. 1ul of saturated cell mixture.
- Plate: http://spreadsheets.google.com/ccc?key=0AvbXytCFRyFCdGJNVXhzNTUtWTgtZFlkYmZGTVQxT2c&hl=en
- by accident, added two uls of different cells to F3
TECAN

Running a TECAN analysis:
The procedure below only works for black 96-well flat bottom plates.
- Turn TECAN power on (wait for light to stop flashing)
- Open XFluor4 Safire II XLS spreadsheet
- Load plate:
- Have 96-well plate with media+cells
- Goto XFluorSafireII menu > movements > out
- Load plate
- Goto XFluorSafireII menu > movements > in
- Load the program to run:
- Select Multi Labeling Kinetic
- Load multi labeling kinetic parameter > "\iGEM 2007\My Documents\Weston\gabe's experimental folder\kinteticmodified_no gfp reading.mps"
- Click "Run"
- Wait for data collection. Operation can only be cancelled when machine is performing measurements.
4/14/2010
Picked 3 colonies from the 3 different constructs. Transformation efficiency is, of course, insane because there was no ligation performed.
 |
 |
 |
Ugh, image markup is confusing. Anyways, refer to the edit page for code to do the above.
4/12/2010
- Trial run for transformation. Obtained vectors.
- jtk2559-jtk2164 K
- jtk2619-jtk2164 K
- jtk2578-jtk2164 K
- Transformation protocol:
- Add:
- plasmid 0.5 uL
- cells (MC1061 pir+) 10 uL
- Add to cells:
- KCM 1.5 uL
- Water 2.5 uL
- Use normal heat shock
- Did @ 42C for 120 sec
- Rescue (rescued 40 min)
- Plate