SBB10AssayTeam1-LabNotebook: Difference between revisions
| Line 8: | Line 8: | ||
1. '''Problem: Absorbances remain unchanged/increases with decreasing protein concentration''' | 1. '''Problem: Absorbances remain unchanged/increases with decreasing protein concentration''' | ||
<blockquote> | <blockquote> | ||
Possible cause: Protein concentrates saturates available antibody | Possible cause: Protein concentrates saturates available antibody<br> | ||
Solution 1: Reduce protein concentrations below 1/10000X<br> | Solution 1: Reduce protein concentrations below 1/10000X<br> | ||
Solution 2: Perform a second purification step | Solution 2: Perform a second purification step<br> | ||
Solution 3: Increase antibody concentrations available to bind protein (primary detecting (anti-His) or primary capture (anti-Myc))<br> | Solution 3: Increase antibody concentrations available to bind protein (primary detecting (anti-His) or primary capture (anti-Myc))<br> | ||
</blockquote> | </blockquote> | ||
Revision as of 14:28, 28 April 2010
4/28/2010
 |
 |
Suggestions for ELISA
1. Problem: Absorbances remain unchanged/increases with decreasing protein concentration
Possible cause: Protein concentrates saturates available antibody
Solution 1: Reduce protein concentrations below 1/10000X
Solution 2: Perform a second purification step
Solution 3: Increase antibody concentrations available to bind protein (primary detecting (anti-His) or primary capture (anti-Myc))
2. Problem: Absorbances remains unchanged/increases with decreasing protein concentration
Possible cause: Protein concentrates saturates available antibody.
Solution 1: Reduce protein concentrations below 1/10000X
Solution 2: Perform a second purification step.
Solution 3: Increase antibody concentrations available to bind protein (primary detecting (anti-His) or primary capture (anti-Myc))
4/26/2010
Did ELISA. Left lab around 5 pm; last step performed was incubating 1h after adding varying concentrations of T4 ligase to 24 wells.
Protocol (roughly):
- Dilute anti-Myc antibody (1000X) to 1X in TBST, plate 100 uL into well
- Incubate at RT for 1h
- Do three plate washes (TBST with 0.1% Tween)
- Put blocking buffer (2% BSA, in TBST) 100 uL in wells, incubate 1h at RT
- Do three plate washes
- Put 100 uL T4 Ligase at various concentrations (diluted in blocking buffer) (see attached spreadsheet) into wells
- Incubate 1h at 37C
- Do three washes
- Put anti-His antibody conjugated to HRP (10000X) 100uL, dilute to 1X in blocking buffer, 1h at 37C
- Do three washes
- Put detection 3,3’,5,5’-Tetramethylbenzidine (TMB) solution 100uL
- Incubate at 37C for 15 min
- Detect with spectrometer at 450 nm
4/22/2010
- Get column, cut tip, tape it to stabilize somewhere high, place waste column beneath the column.
- Pour in lysate + beads
- Collect drips
- Wash with ice cold PBS (20 mL) (N x 0.5 mL) eject PBS against the column wall to wash lysate off the wall.
- Elute with 4-5 times 1 mL volumes of 300 mM imidazole in PBS
- Put eluant into dialysis column, add ice cold PBS to column, then shake in a speed of 5000mph with 4°C for 30 min, then change to fresh PBS, repeat shaking and change media for several times.
- add ligase, purify and run in gel (Mike did this).
4/21/2010
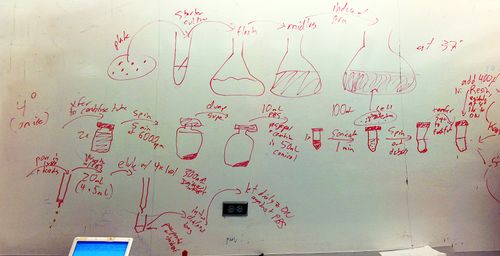
- General protocol for protein purification:
- Pick starter colony
- Grow starter culture
- Put in flask
- Grow to midlog
- Induce arabinose
- Grow to saturation, have protein
- Transfer to centrifuge tube (100 mL) x 2 - stuff here on is on ice (4C)
- Spin 5 min at 6000 rpm
- Dump supernatant, leave pellet
- 10 mL of resuspension buffer (TBS), resuspend and combine in 50 mL conical flask
- Sonicate (probe) - 1 minute (is it a program?). DO pulses.
- Spin to remove debris
- Transfer supernatant to fresh tube (pour). Will be translucent.
- Add 400 uL of Ni resin
- Agitate at 4C 1h to overnight
- Tomorrow
- Get column
- Pour in lysate + beads
- Collect drips
- Wash with PBS (20 mL) (4 x 5 mL)
- Elute with 4-5 1 mL volumes of 300 mM imidazole in PBS
- Put eluant into dialysis bag
- Let dialyze overnight on ice (4°C)
4/19/2010
- analyze TECAN data, maximize TECAN protocol
- TECAN data showed significant edge effects. Recommended to not use outer rows and columns
- I have no clue how to derive the actual growth rate. Tried some logistic equations (a*b)/(a-(b-a)*Exp(-c*t)) and nonlinear regression but my system keeps blowing up, regression looks bad, etc.
- There are a lot of forms of logistic equations you can use. E.x. K / (1 + exp(a + b*x), the differential form dN/dt = r*N(K-N)/K, etc. If anyone has a copy of Mathematica or SPSS, try to play around with it.
- develop ELISA protocol
4/15/2010
- materials: 20% 100X arabinose, LB media and 96 well plate for TECAN
- dilute arabinose to 1X, 50ul LB in each well. 1ul of saturated cell mixture.
- Plate: http://spreadsheets.google.com/ccc?key=0AvbXytCFRyFCdGJNVXhzNTUtWTgtZFlkYmZGTVQxT2c&hl=en
- by accident, added two uls of different cells to F3
TECAN

Running a TECAN analysis:
The procedure below only works for black 96-well flat bottom plates.
- Turn TECAN power on (wait for light to stop flashing)
- Open XFluor4 Safire II XLS spreadsheet
- Load plate:
- Have 96-well plate with media+cells
- Goto XFluorSafireII menu > movements > out
- Load plate
- Goto XFluorSafireII menu > movements > in
- Load the program to run:
- Select Multi Labeling Kinetic
- Load multi labeling kinetic parameter > "\iGEM 2007\My Documents\Weston\gabe's experimental folder\kinteticmodified_no gfp reading.mps"
- Click "Run"
- Wait for data collection. Operation can only be cancelled when machine is performing measurements.
4/14/2010
Picked 3 colonies from the 3 different constructs. Transformation efficiency is, of course, insane because there was no ligation performed.
 |
 |
 |
Ugh, image markup is confusing. Anyways, refer to the edit page for code to do the above.
4/12/2010
- Trial run for transformation. Obtained vectors.
- jtk2559-jtk2164 K
- jtk2619-jtk2164 K
- jtk2578-jtk2164 K
- Transformation protocol:
- Add:
- plasmid 0.5 uL
- cells (MC1061 pir+) 10 uL
- Add to cells:
- KCM 1.5 uL
- Water 2.5 uL
- Use normal heat shock
- Did @ 42C for 120 sec
- Rescue (rescued 40 min)
- Plate