OpenSourceMalaria:Triazolopyrazine (TP) Series: Difference between revisions
Matthew Todd (talk | contribs) (→Strings for Google: adding links to strings) |
Matthew Todd (talk | contribs) (→Strings for Google: more links to strings) |
||
| Line 485: | Line 485: | ||
'''MMV675963''' (OSM-S-271) <br> | '''MMV675963''' (OSM-S-271) <br> | ||
([http://malaria.ourexperiment.org/osm_procedures/9800/Preparation_of_OSMS187.html OSM-S-187]) <br> | ([http://malaria.ourexperiment.org/osm_procedures/9800/Preparation_of_OSMS187.html OSM-S-187]) <br> | ||
([http://malaria.ourexperiment.org/osm_procedures/9804/Preparation_of_OSMS188.html OSM-S-188]) <br> | |||
([http://malaria.ourexperiment.org/osm_procedures/9805/Preparation_of_OSMS189.html OSM-S-189]) <br> | ([http://malaria.ourexperiment.org/osm_procedures/9805/Preparation_of_OSMS189.html OSM-S-189]) <br> | ||
([http://malaria.ourexperiment.org/osm_procedures/9806/Preparation_of_OSMS190.html OSM-S-190]) <br> | |||
([http://malaria.ourexperiment.org/osm_procedures/9807/Preparation_of_OSMS191.html OSM-S-191]) <br> | |||
([http://malaria.ourexperiment.org/osm_procedures/9904/OSMS207.html OSM-S-207]) <br> | |||
([http://malaria.ourexperiment.org/osm_procedures/9907/OSMS208.html OSM-S-208]) <br> | ([http://malaria.ourexperiment.org/osm_procedures/9907/OSMS208.html OSM-S-208]) <br> | ||
([http://malaria.ourexperiment.org/osm_procedures/9963/Preparation_of_OSMS219.html OSM-S-219]) <br> | |||
([http://malaria.ourexperiment.org/osm_procedures/9966/Preparation_of_OSMS220.html OSM-S-220]) <br> | |||
temp | temp | ||
Revision as of 15:38, 18 December 2014
Open Source Malaria Series 4: The Triazolopyrazine (TP) Series
What's New
This series is currently active. (How to respond/input, for example if you want to suggest a molecule that should be made, is described in the Landing Page under "Join the Team")
Automatically updated list of to do items in this Series
Automatically updated list of compounds being made in this series
Most recent online meeting relevant to this series
April 2014 PDF newsletter may be downloaded using this link. Late 2014 newsletter under construction at GHI 269.
Introduction
Preamble
The Triazolopyrazine Series is the newest of the OSM series. It was announced by MMV and on the OSM blog (via the briefing document and as a general description) on September 10th 2013 and is sometimes referred to as the TP Series, or OSM Series 4.

The series arises from industrial work that cannot be fully disclosed which was followed by some hit-to-lead work funded directly by MMV and performed by a CRO which can.
A great deal of exploration of the series has been done, with significant diversity in the core and pendant groups. The series includes many potent compounds, all of which are detailed on this page.
This part of OSM is a lead optimisation project, now aiming to improve solubility, potency and metabolic stability while reducing hERG activity.
As with everything involved in OSM, suggestions can be given in multiple ways.
Start of the Campaign
A briefing document written for MMV was the initial knowledgebase. This was accompanied by a PDF summary of pharmacokinetics and efficacy. This is being folded into the sections below, then supplemented.
The aim of the campaign at the outset was to improve the metabolic stability and the pharmacokinetic properties of this series in rat so as to meet the once-dosing criteria (TCP1) set by MMV. New chemistry directed towards blocking the putative metabolic sites was a major part of the research prior to the data being contributed to OSM.
Sources of Data on the TP Series
Compounds Inherited from MMV including structures and potencies ([http://malaria.ourexperiment.org/osdd_malaria_shared/8106/MMV_triazolopyrazine_data.html
older version of this file])
Initial briefing document (A minor error in the briefing document referring to the amides has been clarified.) Briefing document mostly folded into the below.
PDF summary of pharmacokinetics and efficacy. Needs folding into the below.
The current synthetic lab notebook
Summary of Data on Amides in the Series
Document from CRO describing synthetic chemistry of some members of series
The ELN post containing the raw Chemdraw and picture files for this wiki page - if you generate new pictures, add to the ELN page.
Project reports by Devon Scott and Eduvie Omene, University of Edinburgh
Current Aims
1. Lead optimisation, to improve solubility and metabolic stability while maintaining potency and reducing hERG activity.
2. Validation of PfATP4 activity as mechanism of action.
Current/Recent Activity
Last updated: July 3rd 2014
1. Which compounds to make next? - see the most recent meeting for links to the discussions on these points. Compounds in the ether and amide sub-series are being made. Automatically-updated list of compounds being made now - some compounds may be from older series.
2. Does anyone possess compounds that could be relevant to this series already? It currently appears as though the answer is "no".
3. Resources needed: a) Chemists to make molecules, b) Experienced medicinal chemists to digest the data on this wiki and suggest new compounds to make to improve the pharmacokinetic parameters above. You can contact the OSM Consortium in many ways (go to the "Join the Team" page here.
Notable Points about Series 4
- Compounds in this series have been identified down to 16 nM potency.
- Seems to have good in vitro HLM and hHEP stability CLint < 8.1 is compatible with 10 nM potency.
- RLM remains stubbornly high, particularly for the more potent analogues translating to short half-lives in rat PK. It's possible that rat metabolism may not be a good model for human metabolism for this series.
- Series appears to have little polypharmacology or cytotoxicity.
- There is strong correlation between activity vs. Pfal and activity in Kiaran Kirk’s ion regulation assay, implying the mechanism of action is inhibition of PfATP4.
Concerns about the TP Series
- Although dofetilide binding looks weak or nil, the series has shown activity in a patch clamp assay at Essen (1-10 μM) which is quite potent though with a window of >100 fold over Pfal potency.
- In Kip Guy’s resistant mutants the picture is mixed, but there is still support for the idea that some members of the series are weaker in the resistant strains. The series has no or weak >>1 μM activity against gametocytes, no activity against Winzeler’s Pb liver stage and may have weak activity against ookinetes but the dose-response data has not been completed.
Project Strands of Current Interest
The biggest issue is metabolic stability, as measured in rat in particular. There are few toxicity concerns. Thus possible future directions:
- Small scale changes around the side chains, particularly phenethyl to attempt to balance solubility, potency and metabolism. Other possibilities: a) N is tolerated in the pendant rings, but hasn’t been explored much recently. b) Is 3,4-diF the best substitution pattern? c) Some evidence (eg MMV669848) that the phenethyl side chain can be rigidified, perhaps the iso-indoline of that compound could be improved on with other ring systems and by more optimal substitution of the aromatic benzene ring of the isoindoline. d) The amide MMV670944 is interesting and shows good RLM stability, but many other amides failed to match its potency.
- Incorporation of a basic centre to increase volume as a potential fix for half-life. However, this might come at the expense of plasma concentration so would require high potency. Of the 29 compounds with a basic centre only one (MMV670437, below) has a measured potency < 100 nM (actually 44 nM).

- More significant structural changes. Of the changes made to the basic skeleton, the most successful might be the recent evaluation of the substitution position changes (e.g., MMV670945), possibly in combination with modifying the disposition of the N atoms in the core (though the triazolopyrazine has shown the best data, below).
SAR
Modification of Core Triazolopyrazine
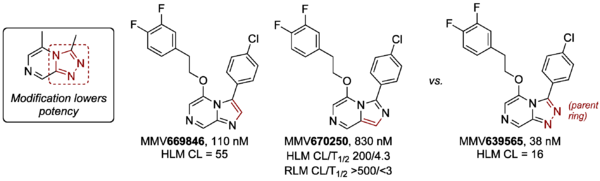
To assess modification of the triazole part of the core, two compounds based on imadazopyrazines were made (MMV669846 and MMV670250). Both showed reduced potency against PfNF54 vs. the corresponding parent compound MMV639565. The RLM stability of MMV670250 was found to be poor.
Modifications to the pyrazine part of the core were not well tolerated. Several aromatic variations were tried.

Several replacements of the aromatic ring with aliphatic rings were assayed.

As most of the analogs arising from core modifications were >1 μM potency, fewer were tested in RLM (though quite a few were tested in HLM). Of the 4 tested in RLM, the greatest stability had a Clint of 109, (HLM 9.5), several had HLM Clint 8 or less, particularly after moving or removing the N from the pyrazine ring. (<--need to locate primary data, since this disagrees with what is in the pictures here - MHT)
Modification of Pyrazine Substitution Pattern
It was thought possible that the pyrazine moiety of the triazolopyrazine could undergo aldehyde oxidase-mediated metabolism at positions alpha- to the nitrogen. Four variations were made to the substitution of the "southernmost" (8-) ring C-H, all lowering potency vs. PfNF54.

Side chain transposition was investigated. The side-chain on the pyrazine ring was shifted to the adjacent carbon. The chain length was varied (n= 0,1,2) and linked through either O or N. Among the ethers, the phenethyl ether MMV670945 showed good potency (Pfal IC50 34 nM) but a poor stability in RLM (Cl 100 mL/min/Kg).
Modification of the Triazole Substitution
Attempts at lowering the lipophilicity of the compounds by replacing the triazole aryl substituent with a cyclo(hetero)aliphatic group, linked either by the heteroatom or otherwise (e.g. piperidine, tetrahydropyran, indoline or isoindoline) lowered the potency against PfNF54, as did an aniline substituent. A dimethylpyrazole substituent was also deleterious - note the comparison with MMV670944, which is potent.
One of the original inherited documents for Series 4 indicated that "heteroaryl" was not tolerated in this position, but the data do not currently support this as a blanket conclusion. Pyridinyl has been found to be a poor substituent, but one inherited compound containing a substituted pyridine was active.

Where an aromatic ring has been used in this position, it appears that a para-OCF3 and -OCHF2 groups generate higher potency than -OCH3.

Pyrazine Side Chain Modifications - Ethers
The phenethyl ether side-chain on the pyrazine ring was flagged as a metabolic hot-spot and so several strategies were adopted to mitigate this risk. Some trends:
- Changing the length of the side-chain to anything other than 3 atoms severely lowers potency against PfNF54
- The linker atom to the pyrazine ring is crucial (O>>C>N)
- Heteroatoms in the side-chain lowered potency with the exception of MMV669848 (not an ether - features an isoindolino-methyl group on the pyrazine ring - see later section) although this compound had poor RLM stability.
- Constraining the linear side-chain into ring systems (e.g, azetidines, pyrrolidines, pyrazoles) severely reduced potency.
A 2-naphthol substituent on the pyrazine ring showed reasonable potency against Pfal (IC50 114 nM) but suffered from poor RLM stability (what is the number?). Hetero-analogs of 2-naphthol e.g. indole, indazole, quinoline, chroman, benzisoxazole, quinazoline, exhibited reduced potency.
In a separate attempt to mitigate the potential metabolism in the ethyl chain it was replaced by an aromatic group through inclusion of a phenol substituent on the pyrazine ring. Some substituted phenolates were metabolically more stable in vitro as well as in vivo in rat, although with reduced potency. (what are the numbers?)
Considering that the benzylic position in the phenethyl side-chain is prone to metabolic oxidation, several compounds having mono- and di-substitution in the benzylic position were made. Di-substitution lowered the potency considerably whereas mono-substitution with OMe, OCHF2, CH2OH, NMe2 groups retained good potency. Additional substitution alpha- to the ether oxygen led to complete loss of potency. The alpha-OCHF2 compound MMV670652, with a p-CN-phenyl group on the triazole ring, showed better RLM stability (cLogP effect). (what is the number?)
Planning of ether analogs: GHI 174 and blog appeal. Planning of more analogs in July 2014: GHI 232.
Pyrazine Side Chain Modifications - Amides
A small library of amides (including the m-Cl benzylamide, MMV668958) showed promising potency. However, other amides (derived from aliphatic or anilines) either showed lower potency or were inactive. The RLM of MMV668958 was poor - perhaps due to benzylic oxidation. Alpha-substitution at the benzylic position or constraining the benzylamine into an aminoindane did not improve potency. Several attempts to make aniline-amides with improved potency against Pfal failed. Loading the aniline ring with lipophilic substituents marginally improved potency but led to poor RLM stability.
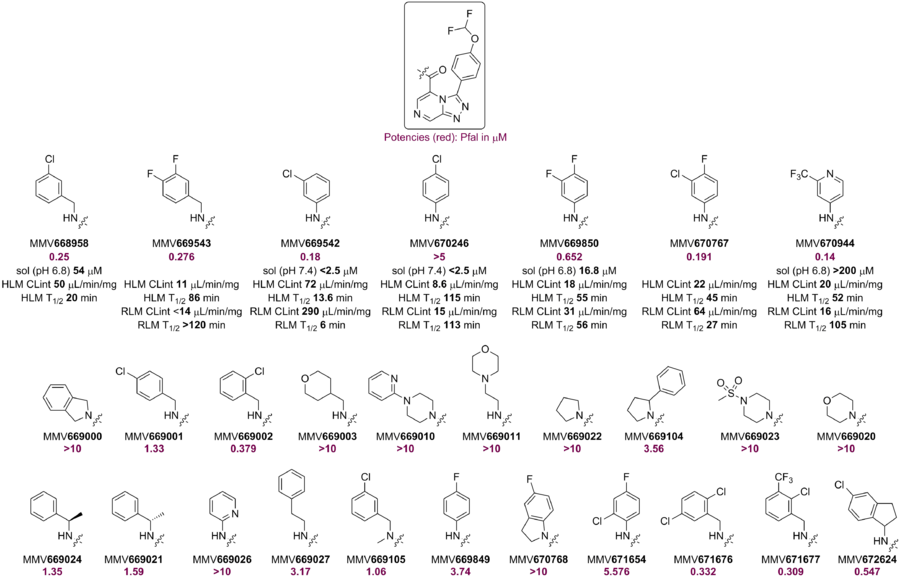
The p-Cl benzanilide MMV670246, although not active against PfNF54, showed good RLM stability perhaps due to lack of benzylic metabolism. However, its rat PK showed high clearance. The potent m-Cl (MMV669542) analog had poor RLM stability.

Planning of amide analogs: GH Issues 110, 101, 123, 207, Joie Garfunkel's suggestions. Planning of more amide analogs in July 2014: GHI 232.
Pyrazine Side Chain Modifications - Others

Physicochem/metabolism/PK
The scheme below shows all the inherited met/PK data inherited, plus additional data for compounds more recently sent to Monash (green). (Associated discussion: GHI 107).
For the more recent set, solubility was still seen as low and needs improving. The metabolic stability measured for the recent set appears not to be a mouse-specific event and needs to be improved to deliver a candidate. Met ID data (below) could help in the design of new analogs with improved stability. Is there a correlation between metabolic stability and Log D?
The PK curve for the relatively weak amide MMV670246 is shown below for oral & IV legs & parameters. The original data are contained in this picture.

A compound with better in vitro balance is MMV670652 but this compound has not been in rat PK. It may be possible to improve potency by synthesis of the more potent enantiomer - this is being addressed through synthesis of the synthetically simpler methyl analog OSM-S-208, the enantioenriched version of which was MMV669844.
The data for MMV639565 were contained in an inherited PDF summary of pharmacokinetics and efficacy.
As would be expected HLM vs. RLM shows a general correlation with approx 4-fold shift on average. However, for most of the more potent analogs, this increases to over 10-fold. The figure below shows the 4 sub 30 nM compounds with HLM & RLM measured: MMV670652, MMV670945, MMV670438 and MMV670947. (It looks like there are several other compounds here exhibiting good metabolic parameters for which we do not have data)

Metabolism ID
MMV669844, MMV669848 and MMV670936 were sent for ID of metabolites and data were obtained.

The data suggest that the core heterocyclic ring is subjected to an oxidation (though it is not clear the extent to which the compound is oxidized, only that certain peaks are present). The nature of the side chain appeared to have little influence on the results, suggesting alteration of the side chain is probably not the way to stop turnover, meaning it is likely that amides made in this series in the near future will also suffer from this problem. Nevertheless the efficacy was seen as sufficiently promising that amides remain attractive as they are. i.e. they are sufficiently robust for this point in the project. Residual question: what is the mechanism of the clearance (oxidation)? Is it CYP mediated? Open action item here. One possible route of metabolism is Aldehyde Oxidase, discussed in the next section.
Aldehyde Oxidase Assay
The possibility that the compounds were cleared by aldehyde oxidase was discussed (GHI 214), compounds were sent to Pfizer for evaluation and data returned from Scott Obach's laboratory (along with comments from him), summarized below, indicating that some Series 4 compounds were substrates for AO, and others are not.
In Vivo Efficacy
MMV639565 and MMV669844 have both demonstrated parasite clearance in vivo, providing a highly attractive feature of this series.

The therapeutic efficacy of MMV639565 against P falciparum growing in peripheral blood of NODscidIL2Rγnull mice engrafted with human erythrocytes (po dosing, qd for 4 days) is shown below. A rapid parasite clearance - ED90 6.3 mg/kg was recorded.

Values in brackets indicate dose corrected according to quality control of formulation.
The data for MMV669844 show 99.9% inhibition of parasitemia in a snapshot (one dose level of 4 x 50 mg/kg).
Other Observations
Lipophilicity & lipophilic efficiency:
Few compounds achieve a lipophilic efficiency (as measured by pIC50 – AlogP) of greater than 4.0

Mechanism of Action: Possible PfATP4 Activity Deduced from Parasite Ion Regulation Assays
The following five compounds were evaluated in parasite ion regulation assays in the Kirk Laboratory; the hypothesis is that PfATP4 is a Na+ ATPase that exports Na+ and imports H+ (or equivalent) and that the effects of the compounds on Na+ concentration and pH are attributable to inhibition of this activity. Structures, potency, metabolism/solubility and raw PfATP4 assay data are here.

MMV669000: no (potency: inactive)
MMV669304: yes (potency: 280 nM)
MMV669360: yes (potency: 356 nM)
MMV669542: yes (potency: 185 nM)
MMV669848: yes (potency: 114 nM)
MMV669000 did not dissipate the plasma membrane Na+ gradient or increase the plasma membrane pH gradient consistent with it not inhibiting PfATP4 at the concentration tested.
The other compounds dissipated the plasma membrane Na+ gradient and increased the plasma membrane pH gradient at a concentration of 2 μM, consistent with them being PfATP4 inhibitors.
(i.e. note the correlation: compound inactive in these assays is the inactive analog in the plasmodium screen)
Testing was carried out vs. PfATP4-resistant mutants (ELN entry and GHI 251) in the laboratories of Kiaran Kirk and David Fidock.
hERG Activity
MMV669844 and MMV670944 (OSM-S-175) were evaluated in a hERG assay, with the data indicating issues with both compounds that will need to be addressed. To investigate whether this is a problem for the series, several compounds (inherited and new, attempting to reduce cLogP - GH Issue #211) were evaluated for activity by AstraZeneca using this assay to see if this activity can be reduced. The results suggest that either the pendant OCHF2 or the amide is a problem. In contrast the previous two data points suggest that hERG activity can be obtained with compounds containing neither feature. Future analogs will need to address this. Cumulative data shown below (from here).

Former OSM postdoc Murray Robertson is applying literature hERG pharmacophore models to the series (GH Issue #188), and a community appeal is active for a laboratory willing to run higher-throughput binding assays (GH Issue #192). There is a lot of literature on hERG models (i.e. how to reduce hERG activity) with the most recent being an analysis of the ChEMBL database concluding that the dominant influence remains lipophilicity.
Toxicity
Toxicity has been evaluated at various points for this series and has generally been found to be low. e.g. here
Synthetic Chemistry
Synthetic Design
The approach to the synthesis of Series 4 up has mainly involved the functionalization of a pyrazine core, followed by a cyclization reaction to give the triazolopyrazine which may then be subsequently functionalized. The two main series, ether- and amide-linked compounds are shown below along with a specific example of a potent compound in each series.

Synthesis of the Ether-Linked Series
The synthesis of members of the ether series has been largely solved by Jo Ubels, as shown below.
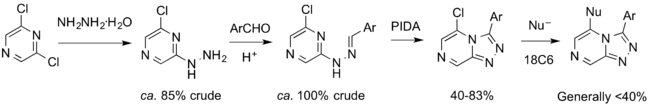
The cyclisation was achieved, initially not in the ca. 50% yields reported by the original CRO. Jo Ubels has solved this, with input from others such as a student working with Patrick Thomson, Devon Scott.
The union of the alcohol fragment with the chloro-intermediate was for a long time not proceeding cleanly, and this was also found by the CRO that first reported this route. Jo Ubels' lit survey found conditions involving a crown ether that has worked reliably. There have been periodic discussions about which alcohols to employ in the resulting synthetic scheme (post from April 2014, GHI174).
A synthetic challenge was the efficiency of the oxidative cyclization to form the triazolopyrazine. There was much discussion of the use of oxidants such as PIDA and chloramine T (Overview GHI 206) as well as the possibility that hydrazone isomers were cyclising at different rates (both Github Issue 97).
Some of the ether compounds contain a stereogenic centre in the benzylic position - structures are in the SAR section above, and the syntheses of these are dealt with below in the section of stereochemistry.
Synthesis of the Amide-Linked Series
Route 1: Initial Amide Bond Formation: It was decided (GHI121, GHI101) to pursue a route involving initial amide bond formation with the chloro-pyrazine acid shown below (OSM-S-150). This molecule is commercially available but is expensive, but there are now routes to it in the lab. Patrick and Inga planned this synthesis and then Inga completed it, as has Eduvie using the same approach (data missing in the lab notebook, but reports are available). [http://malaria.ourexperiment.org/triazolopyrazine_se/9189 Tom scaled up the Patrick/Eduvie/Inga approach and has been completed on a 15 g scale in four steps: 1 2 3 4. An assessment of the relative costs of synthesis vs. purchase was discussed (GHI163, GHI164) but not properly completed.

Sabin has successfully demonstrated an elegant alternative route to the starting pyrazine acid in one step from the methyl precursor (data).
For the subsequent coupling of the pyrazine acid with an amine, Tom and Alice have identified reliable conditions for the amide coupling using T3P that worked much better than an attempt to go via the acid chloride. There was an analysis of commercially-available primary and secondary amines that could be employed, the likely physical properties of the resulting compounds as well as an analysis of those amines available locally.
Hydrazone synthesis was surveyed by Inga) and is working reliably. (Discussion about making hydrazone first, then coupling with pyrazine: GHI152) Following work by Jo Ubels on optimizing the cyclization step for the ether series (above), hypervalent iodide PIDA may now be used to form the triazolopyrazine, as in this example.
Route 2: Carbonylation An efficient alternative synthetic approach would be to use the same chloro-triazolopyrazine core as for the ether series (above) and invent a new carbonylation method to introduce the amide as the final sequence (GHI101, GHI126, Inga's lit survey, Alice's original appeal for a collaborator, a summary of the associated discussion, Alice's framing of the relevant reactions (GHI205) and Tom's lit survey. The most recent results (GHI259) indicate there is a significant competing SNAr reaction when an alcohol was used as a nucleophile. The most recent reaction to date in this series was in Sept 2014.
Alternative Routes to the Triazolopyrazine Core
A route permitting more late-stage diversification of the core would involve synthesis of a halogenated core that could be subjected to cross-coupling or nucleophilic displacement. Two such routes are shown below.

Preliminary progress towards both has been made by Tom Macdonald (link to Honours thesis coming). The first steps (acid-mediated cyclizations) have been successfully carried out for Halogenation Routes 1 and 2. The bromination step in Route 1 has reportedly been carried out (we lack the experimental write-up) and the halogenation in Route 2 was reportedly carried out in 58% yield (most recent lab book entry of presumably different attempt, more recent attempt by Alice).
Fluoroalkene Isostere
Jo Ubels suggested a fluoroalkene isostere for the amide series. Jo pursued a simplified version of this chemistry with a pyridine ring in place of the pyrazine. The boronic ester (rather than the acids, which were predicted and found to be unstable, GHI250) was synthesized along with the bromofluoroalkene, but the coupling of these fragments has not yet been well explored. (Link to Ubels Honours thesis coming). Most recent experiment in this series.

Spectroscopy
Suggestion of the study of aryl-aryl interactions, with lit: GHI 232.
Chirality/Stereogenic Centres in This Series
Racemates/Resolutions: Frontrunner compound MMV670652 was evaluated/inherited as a racemate. There was interest in separating the enantiomers (GHI115, GHI111, GHI120. However, the difluoromethyl group makes the compound slightly challenging to synthesize (GHI119 and blog post). It is not clear that this group is needed - there are related structures that could be tried, and compounds lacking this group (and indeed the whole stereogenic centre) are still active as shown in the scheme. MMV669844 (containing CH3 rather than CHF2) was an inherited compound, and was reportedly synthesized using a Sharpless epoxidation on the precursor styrene, but there are no data on the enantiomeric excess of the product - it is shown as a single enantiomer in the original briefing document, so it is presumed (only) that the sample possessed an ee. Compound OSM-S-208 (GHI166), identical to MMV669844 save that it was synthesized as a racemate, was found to be reasonably potent and can therefore be used as a surrogate for MMV670652 for the separation of enantiomers in this series (GHI111). (Note there is ongoing discussion about whether compounds differing in ee, including where the ee is not known, should have different codes (GHI172)).

Alternatives: The CF3-substituted compound shown has yet to be made but has a prohibitively high cLogP; however the hydroxyl-substituted compound remains of interest (GHI178) and the des-F analog is under investigation (GHI235). MMV675948 (OSM-S-265) was found to be reasonably potent, providing another simple analog suitable for enantiomer separation. GHI236). Methoxy-substitured ether analogs lacking the Ph in the side chain are under investigation (GHI238, GHI239) though the only related inherited analog (MMV670762) is inactive.
Generally speaking all inherited compounds should be treated as racemates unless there is reason not to.
One approach to the synthesis of the chiral alcohol fragment needed for the racemic samples (e.g. of MMV670652) requires a source of cyanide.
Another approach to enantioenriched compounds in this series is through benzylic amines, either the known compounds MMV670437, MMV670763 and MMV671651 or analogs thereof (see GHI242, GHI241 and GHI240 respectively. (All three inherited amines were supposedly enantioenriched).

Enantioselective Approaches:
Alice Williamson proposed an enantioselective approach to the benzylic-functionalized ether compounds (GHI167).
Other Sources of Compounds Relevant to this Series
(Original community request)
The compound series appears to be novel/unusual (related: GHI142) with little similarity to compounds in PubChem/Surechem, though Chris Southan (in the previous link) found a related compound (CID 7118230) with reported antimalarial activity, with a structure similar to those described above for modification of the triazolopyrazine core. Jo Ubels also carried out a search and found few compounds similar to Series 4.
A similarity was noted between Series 4 compounds and a compound from Novartis likely active against vivax. Paul Willis suggested the mechanism of action of the Novartis compounds was likely PI4K - data and reasoning given in this lab book entry. Chris Southan modelled the OSM and Novartis compounds, which provides additional evidence for a different mechanism of action.
Desirable Compounds Not Yet Synthesised
Side chain derived from phenylalanine: GHI 254, ELN
ClC(C=C1)=CC=C1C2=NN=C3C=NC=C(OC[C@H](N)CC4=CC=CC=C4)N32 InChI=1S/C20H18ClN5O/c21-16-8-6-15(7-9-16)20-25-24-18-11-23-12-19(26(18)20)27-13-17(22)10-14-4-2-1-3-5-14/h1-9,11-12,17H,10,13,22H2/t17-/m1/s1
Strings for Google
Use this section to paste strings to make the page more discoverable.
MMV639565 FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(C4=CC=C(Cl)C=C4)N32)=C1 InChI=1S/C19H13ClF2N4O/c20-14-4-2-13(3-5-14)19-25-24-17-10-23-11-18(26(17)19)27-8-7-12-1-6-15(21)16(22)9-12/h1-6,9-11H,7-8H2
MMV639725 (OSM-S-189)
MMV668822 FC1=C(F)C=CC(CCOC2=C[N+]([O-])=CC3=NN=C(C4=CC=C(OC(F)F)C=C4)N32)=C1 InChI=1S/C20H14F4N4O3/c21-15-6-1-12(9-16(15)22)7-8-30-18-11-27(29)10-17-25-26-19(28(17)18)13-2-4-14(5-3-13)31-20(23)24/h1-6,9-11,20H,7-8H2
MMV668823 FC1=C(F)C=CC(CCOC2=CN=C(Cl)C3=NN=C(C4=CC=C(OC(F)F)C=C4)N32)=C1 InChI=1S/C20H13ClF4N4O2/c21-17-19-28-27-18(12-2-4-13(5-3-12)31-20(24)25)29(19)16(10-26-17)30-8-7-11-1-6-14(22)15(23)9-11/h1-6,9-10,20H,7-8H2
MMV668824 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CCO)N32 InChI=1S/C14H12F2N4O2/c15-14(16)22-11-3-1-9(2-4-11)13-19-18-12-8-17-7-10(5-6-21)20(12)13/h1-4,7-8,14,21H,5-6H2
MMV668955 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C(CCO)CNC3 InChI=1S/C14H16F2N4O2/c15-14(16)22-11-3-1-9(2-4-11)13-19-18-12-8-17-7-10(5-6-21)20(12)13/h1-4,10,14,17,21H,5-8H2
MMV668956 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4CN(C5=CC(F)=C(F)C=C5)C4 InChI=1S/C21H15F4N5O2/c22-16-6-3-13(7-17(16)23)29-10-15(11-29)31-19-9-26-8-18-27-28-20(30(18)19)12-1-4-14(5-2-12)32-21(24)25/h1-9,15,21H,10-11H2
MMV668957 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(N4CCC(C5=CC=CC=C5)C4)N32 InChI=1S/C22H19F2N5O/c23-22(24)30-18-8-6-16(7-9-18)21-27-26-19-12-25-13-20(29(19)21)28-11-10-17(14-28)15-4-2-1-3-5-15/h1-9,12-13,17,22H,10-11,14H2
MMV668958 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C(C(NCC4=CC(Cl)=CC=C4)=O)=CN=C3 InChI=1S/C20H14ClF2N5O2/c21-14-3-1-2-12(8-14)9-25-19(29)16-10-24-11-17-26-27-18(28(16)17)13-4-6-15(7-5-13)30-20(22)23/h1-8,10-11,20H,9H2,(H,25,29)
MMV668959 FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(C4CCOCC4)N32)=C1 InChI=1S/C18H18F2N4O2/c19-14-2-1-12(9-15(14)20)3-8-26-17-11-21-10-16-22-23-18(24(16)17)13-4-6-25-7-5-13/h1-2,9-11,13H,3-8H2
MMV668960 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2CCNC3 InChI=1S/C12H12F2N4O/c13-12(14)19-9-3-1-8(2-4-9)11-17-16-10-7-15-5-6-18(10)11/h1-4,12,15H,5-7H2
MMV668961 FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(C4CCN(C(C)=O)CC4)N32)=C1 InChI=1S/C20H21F2N5O2/c1-13(28)26-7-4-15(5-8-26)20-25-24-18-11-23-12-19(27(18)20)29-9-6-14-2-3-16(21)17(22)10-14/h2-3,10-12,15H,4-9H2,1H3
MMV668962 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2CCN(C(CN)=O)C3 InChI=1S/C14H15F2N5O2/c15-14(16)23-10-3-1-9(2-4-10)13-19-18-11-8-20(12(22)7-17)5-6-21(11)13/h1-4,14H,5-8,17H2
MMV669000 O=C(N1CC(C=CC=C2)=C2C1)C3=CN=CC4=NN=C(C5=CC=C(OC(F)F)C=C5)N43 InChI=1S/C21H15F2N5O2/c22-21(23)30-16-7-5-13(6-8-16)19-26-25-18-10-24-9-17(28(18)19)20(29)27-11-14-3-1-2-4-15(14)12-27/h1-10,21H,11-12H2
MMV669001 in amide scheme
MMV669002 in amide scheme
MMV669003 in amide scheme
MMV669006 FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(NC4=CC=C(F)C(F)=C4)N32)=C1 InChI=1S/C19H13F4N5O/c20-13-3-1-11(7-15(13)22)5-6-29-18-10-24-9-17-26-27-19(28(17)18)25-12-2-4-14(21)16(23)8-12/h1-4,7-10H,5-6H2,(H,25,27)
MMV669008 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(N4CC(OC5=CC(F)=C(F)C=C5)C4)N32 InChI=1S/C21H15F4N5O2/c22-16-6-5-14(7-17(16)23)31-15-10-29(11-15)19-9-26-8-18-27-28-20(30(18)19)12-1-3-13(4-2-12)32-21(24)25/h1-9,15,21H,10-11H2
MMV669009 FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(N4CCCCC4)N32)=C1 InChI=1S/C18H19F2N5O/c19-14-5-4-13(10-15(14)20)6-9-26-17-12-21-11-16-22-23-18(25(16)17)24-7-2-1-3-8-24/h4-5,10-12H,1-3,6-9H2
MMV669010 in amide scheme
MMV669011 in amide scheme
MMV669020 in amide scheme
MMV669021 in amide scheme
MMV669022 in amide scheme
MMV669023 in amide scheme
MMV669024 in amide scheme
MMV669025 FC1=C(F)C=CC(CCOC2=CNC(C3=NN=C(C4=CC=C(OC(F)F)C=C4)N32)=O)=C1 InChI=1S/C20H14F4N4O3/c21-14-6-1-11(9-15(14)22)7-8-30-16-10-25-19(29)18-27-26-17(28(16)18)12-2-4-13(5-3-12)31-20(23)24/h1-6,9-10,20H,7-8H2,(H,25,29)
MMV669026 in amide scheme
MMV669027 in amide scheme
MMV669028 FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(N4CC(C=CC=C5)=C5C4)N32)=C1 InChI=1S/C21H17F2N5O/c22-17-6-5-14(9-18(17)23)7-8-29-20-11-24-10-19-25-26-21(28(19)20)27-12-15-3-1-2-4-16(15)13-27/h1-6,9-11H,7-8,12-13H2
MMV669102 FC1=C(F)C=CC(CCOC2=CN=C(N(CC)CC)C3=NN=C(C4=CC=C(OC(F)F)C=C4)N32)=C1 InChI=1S/C24H23F4N5O2/c1-3-32(4-2)22-23-31-30-21(16-6-8-17(9-7-16)35-24(27)28)33(23)20(14-29-22)34-12-11-15-5-10-18(25)19(26)13-15/h5-10,13-14,24H,3-4,11-12H2,1-2H3
MMV669103 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(NS(CC4=CC=C(C(F)(F)F)C=C4)(=O)=O)N32 InChI=1S/C20H14F5N5O3S/c21-19(22)33-15-7-3-13(4-8-15)18-28-27-16-9-26-10-17(30(16)18)29-34(31,32)11-12-1-5-14(6-2-12)20(23,24)25/h1-10,19,29H,11H2
MMV669104 in amide scheme
MMV669105 in amide scheme
MMV669304 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CCCC4=CC=CC=C4)N32 InChI=1S/C21H18F2N4O/c22-21(23)28-18-11-9-16(10-12-18)20-26-25-19-14-24-13-17(27(19)20)8-4-7-15-5-2-1-3-6-15/h1-3,5-6,9-14,21H,4,7-8H2
MMV669305 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CCN4CCOCC4)N32 InChI=1S/C18H19F2N5O2/c19-18(20)27-15-3-1-13(2-4-15)17-23-22-16-12-21-11-14(25(16)17)5-6-24-7-9-26-10-8-24/h1-4,11-12,18H,5-10H2
MMV669310 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C(CCCC4=CC=CC=C4)CNC3 InChI=1S/C21H22F2N4O/c22-21(23)28-18-11-9-16(10-12-18)20-26-25-19-14-24-13-17(27(19)20)8-4-7-15-5-2-1-3-6-15/h1-3,5-6,9-12,17,21,24H,4,7-8,13-14H2
MMV669311 FC1=C(F)C=CC(CCOC2=CN=C(N)C3=NN=C(C4=CC=C(OC(F)F)C=C4)N32)=C1 InChI=1S/C20H15F4N5O2/c21-14-6-1-11(9-15(14)22)7-8-30-16-10-26-17(25)19-28-27-18(29(16)19)12-2-4-13(5-3-12)31-20(23)24/h1-6,9-10,20H,7-8H2,(H2,25,26)
MMV669312 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CCN4CC(C=CC=C5)=C5C4)N32 InChI=1S/C22H19F2N5O/c23-22(24)30-19-7-5-15(6-8-19)21-27-26-20-12-25-11-18(29(20)21)9-10-28-13-16-3-1-2-4-17(16)14-28/h1-8,11-12,22H,9-10,13-14H2
MMV669353 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(N4CC(C5=CC=CC=C5)C4)N32 InChI=1S/C21H17F2N5O/c22-21(23)29-17-8-6-15(7-9-17)20-26-25-18-10-24-11-19(28(18)20)27-12-16(13-27)14-4-2-1-3-5-14/h1-11,16,21H,12-13H2
MMV669360 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(COCC4=CC=C(F)C(F)=C4)N32 InChI=1S/C20H14F4N4O2/c21-16-6-1-12(7-17(16)22)10-29-11-14-8-25-9-18-26-27-19(28(14)18)13-2-4-15(5-3-13)30-20(23)24/h1-9,20H,10-11H2
MMV669541 FC1=C(F)C=CC(CCOC2=CN=C(C)C3=NN=C(C4=CC=C(OC(F)F)C=C4)N32)=C1 InChI=1S/C21H16F4N4O2/c1-12-19-27-28-20(14-3-5-15(6-4-14)31-21(24)25)29(19)18(11-26-12)30-9-8-13-2-7-16(22)17(23)10-13/h2-7,10-11,21H,8-9H2,1H3
MMV669542 (OSM-S-202) FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(C(NC4=CC=CC(Cl)=C4)=O)N32 InChI=1S/C19H12ClF2N5O2/c20-12-2-1-3-13(8-12)24-18(28)15-9-23-10-16-25-26-17(27(15)16)11-4-6-14(7-5-11)29-19(21)22/h1-10,19H,(H,24,28)
MMV669543 in amide scheme
MMV669544 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CNC(C4=CC(Cl)=CC=C4)=O)N32 InChI=1S/C20H14ClF2N5O2/c21-14-3-1-2-13(8-14)19(29)25-10-15-9-24-11-17-26-27-18(28(15)17)12-4-6-16(7-5-12)30-20(22)23/h1-9,11,20H,10H2,(H,25,29)
MMV669784 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(Cl)C=C4 InChI=1S/C18H11ClF2N4O2/c19-12-3-7-13(8-4-12)26-16-10-22-9-15-23-24-17(25(15)16)11-1-5-14(6-2-11)27-18(20)21/h1-10,18H
MMV669844 [C@H](COc1cncc2n1c(nn2)c1ccc(cc1)C#N)(c1cc(c(cc1)F)F)OC
MMV669846 FC1=C(F)C=CC(CCOC2=CN=CC3=NC=C(C4=CC=C(Cl)C=C4)N32)=C1 InChI=1S/C20H14ClF2N3O/c21-15-4-2-14(3-5-15)18-10-25-19-11-24-12-20(26(18)19)27-8-7-13-1-6-16(22)17(23)9-13/h1-6,9-12H,7-8H2
MMV669848 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CN4CC(C=CC=C5)=C5C4)N32 InChI=1S/C21H17F2N5O/c22-21(23)29-18-7-5-14(6-8-18)20-26-25-19-10-24-9-17(28(19)20)13-27-11-15-3-1-2-4-16(15)12-27/h1-10,21H,11-13H2
MMV669849 in amide scheme
MMV669850 in amide scheme
MMV670243 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(CCCC4=CC(F)=C(F)C=C4)N32 InChI=1S/C21H16F4N4O/c22-17-9-4-13(10-18(17)23)2-1-3-15-11-26-12-19-27-28-20(29(15)19)14-5-7-16(8-6-14)30-21(24)25/h4-12,21H,1-3H2
MMV670246 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C(C(NC4=CC=C(Cl)C=C4)=O)=CN=C3 InChI=1S/C19H12ClF2N5O2/c20-12-3-5-13(6-4-12)24-18(28)15-9-23-10-16-25-26-17(27(15)16)11-1-7-14(8-2-11)29-19(21)22/h1-10,19H,(H,24,28)
MMV670249 FC1=C(F)C=CC(CCOC2=CN=CC3=NN=C(N4CCC5=C4C=CC(F)=C5)N32)=C1 InChI=1S/C21H16F3N5O/c22-15-2-4-18-14(10-15)5-7-28(18)21-27-26-19-11-25-12-20(29(19)21)30-8-6-13-1-3-16(23)17(24)9-13/h1-4,9-12H,5-8H2
MMV670250 FC1=C(F)C=CC(CCOC2=CN=CC3=CN=C(C4=CC=C(Cl)C=C4)N32)=C1 InChI=1S/C20H14ClF2N3O/c21-15-4-2-14(3-5-15)20-25-11-16-10-24-12-19(26(16)20)27-8-7-13-1-6-17(22)18(23)9-13/h1-6,9-12H,7-8H2
MMV670437 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(OC[C@H](N(C)C)C4=CC(F)=C(F)C=C4)N32 InChI=1S/C22H19F4N5O2/c1-30(2)18(14-5-8-16(23)17(24)9-14)12-32-20-11-27-10-19-28-29-21(31(19)20)13-3-6-15(7-4-13)33-22(25)26/h3-11,18,22H,12H2,1-2H3/t18-/m0/s1
MMV670438 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC4(COC4)C5=CC=C(F)C(F)=C5 InChI=1S/C22H16F4N4O3/c23-16-6-3-14(7-17(16)24)22(10-31-11-22)12-32-19-9-27-8-18-28-29-20(30(18)19)13-1-4-15(5-2-13)33-21(25)26/h1-9,21H,10-12H2
MMV670652 FC(F)OC(C1=CC(F)=C(F)C=C1)COC2=CN=CC3=NN=C(C4=CC=C(C#N)C=C4)N32 InChI=1S/C21H13F4N5O2/c22-15-6-5-14(7-16(15)23)17(32-21(24)25)11-31-19-10-27-9-18-28-29-20(30(18)19)13-3-1-12(8-26)2-4-13/h1-7,9-10,17,21H,11H2
MMV670659 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C=CC=C5)C5=C4 InChI=1S/C22H14F2N4O2/c23-22(24)30-17-8-6-15(7-9-17)21-27-26-19-12-25-13-20(28(19)21)29-18-10-5-14-3-1-2-4-16(14)11-18/h1-13,22H
MMV670762 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC(F)(F)F InChI=1S/C14H9F5N4O2/c15-13(16)25-9-3-1-8(2-4-9)12-22-21-10-5-20-6-11(23(10)12)24-7-14(17,18)19/h1-6,13H,7H2
MMV670763 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC[C@H](NC)C4=CC=C(F)C(F)=C4 InChI=1S/C21H17F4N5O2/c1-26-17(13-4-7-15(22)16(23)8-13)11-31-19-10-27-9-18-28-29-20(30(18)19)12-2-5-14(6-3-12)32-21(24)25/h2-10,17,21,26H,11H2,1H3/t17-/m0/s1
MMV670764 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C(F)(F)F)C=C4 InChI=1S/C19H11F5N4O2/c20-18(21)30-14-5-1-11(2-6-14)17-27-26-15-9-25-10-16(28(15)17)29-13-7-3-12(4-8-13)19(22,23)24/h1-10,18H
MMV670765 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(OC4=CC(Cl)=C(Cl)C=C4)N32 InChI=1S/C18H10Cl2F2N4O2/c19-13-6-5-12(7-14(13)20)27-16-9-23-8-15-24-25-17(26(15)16)10-1-3-11(4-2-10)28-18(21)22/h1-9,18H
MMV670767 in amide scheme
MMV670768 in amide scheme
MMV670934 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(NC4=CC(Cl)=C(Cl)C=C4)N32 InChI=1S/C18H11Cl2F2N5O/c19-13-6-3-11(7-14(13)20)24-15-8-23-9-16-25-26-17(27(15)16)10-1-4-12(5-2-10)28-18(21)22/h1-9,18,24H
MMV670935 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=CC(Cl)=C4 InChI=1S/C18H11ClF2N4O2/c19-12-2-1-3-14(8-12)26-16-10-22-9-15-23-24-17(25(15)16)11-4-6-13(7-5-11)27-18(20)21/h1-10,18H
MMV670936 C(COc1cncc2n1c(nn2)c1cnc(cc1)C(F)(F)F)c1cc(c(cc1)F)F
MMV670944 (OSM-S-175) FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C(C(NC4=CC=NC(F)=C4)=O)=CN=C3 InChI=1S/C18H11F3N6O2/c19-14-7-11(5-6-23-14)24-17(28)13-8-22-9-15-25-26-16(27(13)15)10-1-3-12(4-2-10)29-18(20)21/h1-9,18H,(H,23,24,28)
MMV670945 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(OCCC4=CC=C(F)C(F)=C4)N=C3 InChI=1S/C20H14F4N4O2/c21-15-6-1-12(9-16(15)22)7-8-29-18-11-28-17(10-25-18)26-27-19(28)13-2-4-14(5-3-13)30-20(23)24/h1-6,9-11,20H,7-8H2
MMV670946 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(OCC4=CC=C(F)C(F)=C4)N=C3 InChI=1S/C19H12F4N4O2/c20-14-6-1-11(7-15(14)21)10-28-17-9-27-16(8-24-17)25-26-18(27)12-2-4-13(5-3-12)29-19(22)23/h1-9,19H,10H2
MMV670947 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC(CO)C4=CC=C(F)C(F)=C4 InChI=1S/C21H16F4N4O3/c22-16-6-3-13(7-17(16)23)14(10-30)11-31-19-9-26-8-18-27-28-20(29(18)19)12-1-4-15(5-2-12)32-21(24)25/h1-9,14,21,30H,10-11H2
MMV670949 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(NCC4=CC=C(F)C(F)=C4)N=C3 InChI=1S/C19H13F4N5O/c20-14-6-1-11(7-15(14)21)8-24-16-10-28-17(9-25-16)26-27-18(28)12-2-4-13(5-3-12)29-19(22)23/h1-7,9-10,19,24H,8H2
MMV671647 FC(C(F)=C1)=CC=C1[C@@H](N2CCOCC2)COC3=CN=CC4=NN=C(C5=CC=C(OC(F)F)C=C5)N43 InChI=1S/C24H21F4N5O3/c25-18-6-3-16(11-19(18)26)20(32-7-9-34-10-8-32)14-35-22-13-29-12-21-30-31-23(33(21)22)15-1-4-17(5-2-15)36-24(27)28/h1-6,11-13,20,24H,7-10,14H2/t20-/m0/s1
MMV671648 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C(C)(C)C)C=C4 InChI=1S/C22H20F2N4O2/c1-22(2,3)15-6-10-16(11-7-15)29-19-13-25-12-18-26-27-20(28(18)19)14-4-8-17(9-5-14)30-21(23)24/h4-13,21H,1-3H3
MMV671649 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(CCCC5)C5=C4 InChI=1S/C22H18F2N4O2/c23-22(24)30-17-8-6-15(7-9-17)21-27-26-19-12-25-13-20(28(19)21)29-18-10-5-14-3-1-2-4-16(14)11-18/h5-13,22H,1-4H2
MMV671650 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC(C(F)(F)F)=C(Cl)C=C4 InChI=1S/C19H10ClF5N4O2/c20-14-6-5-12(7-13(14)19(23,24)25)30-16-9-26-8-15-27-28-17(29(15)16)10-1-3-11(4-2-10)31-18(21)22/h1-9,18H
MMV671651 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC[C@H](N)C4=CC=C(F)C(F)=C4 InChI=1S/C20H15F4N5O2/c21-14-6-3-12(7-15(14)22)16(25)10-30-18-9-26-8-17-27-28-19(29(17)18)11-1-4-13(5-2-11)31-20(23)24/h1-9,16,20H,10,25H2/t16-/m0/s1
MMV671652 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(N[C@H](C4=CC=C(F)C(F)=C4)CO)N32 InChI=1S/C20H15F4N5O2/c21-14-6-3-12(7-15(14)22)16(10-30)26-17-8-25-9-18-27-28-19(29(17)18)11-1-4-13(5-2-11)31-20(23)24/h1-9,16,20,26,30H,10H2/t16-/m0/s1
MMV671653 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(N4N=CC(C5=CC(F)=C(F)C=C5)=C4)N32 InChI=1S/C21H12F4N6O/c22-16-6-3-13(7-17(16)23)14-8-27-30(11-14)19-10-26-9-18-28-29-20(31(18)19)12-1-4-15(5-2-12)32-21(24)25/h1-11,21H
MMV671654 in amide scheme
MMV671655 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(NC4=CC=C(F)C(F)=C4)N=C3 InChI=1S/C18H11F4N5O/c19-13-6-3-11(7-14(13)20)24-15-9-27-16(8-23-15)25-26-17(27)10-1-4-12(5-2-10)28-18(21)22/h1-9,18,24H
MMV671676 in amide scheme
MMV671677 in amide scheme
MMV671678 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(N4C=CN=C4CC5=CC(F)=C(F)C=C5)N32 InChI=1S/C22H14F4N6O/c23-16-6-1-13(9-17(16)24)10-18-28-7-8-31(18)20-12-27-11-19-29-30-21(32(19)20)14-2-4-15(5-3-14)33-22(25)26/h1-9,11-12,22H,10H2
MMV671679 CC1=NN(C2=CC=C(F)C=C2)C(C)=C1C3=NN=C4C=NC=C(OCC(F)(F)F)N43 InChI=1S/C18H14F4N6O/c1-10-16(11(2)28(26-10)13-5-3-12(19)4-6-13)17-25-24-14-7-23-8-15(27(14)17)29-9-18(20,21)22/h3-8H,9H2,1-2H3
MMV671680 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(S(F)(F)(F)(F)F)C=C4 InChI=1S/C18H11F7N4O2S/c19-18(20)31-13-3-1-11(2-4-13)17-28-27-15-9-26-10-16(29(15)17)30-12-5-7-14(8-6-12)32(21,22,23,24)25/h1-10,18H
MMV671926 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(OC4=CC=C(F)C(F)=C4)N=C3 InChI=1S/C18H10F4N4O2/c19-13-6-5-12(7-14(13)20)27-16-9-26-15(8-23-16)24-25-17(26)10-1-3-11(4-2-10)28-18(21)22/h1-9,18H
MMV671927 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(NCCC4=CC=C(F)C(F)=C4)N=C3 InChI=1S/C20H15F4N5O/c21-15-6-1-12(9-16(15)22)7-8-25-17-11-29-18(10-26-17)27-28-19(29)13-2-4-14(5-3-13)30-20(23)24/h1-6,9-11,20,25H,7-8H2
MMV671929 CC1=NN(C2=CC=C(F)C=C2)C(C)=C1C3=NN=C4C=NC=C(OC5=CC=C(Cl)C(Cl)=C5)N43 InChI=1S/C22H15Cl2FN6O/c1-12-21(13(2)31(29-12)15-5-3-14(25)4-6-15)22-28-27-19-10-26-11-20(30(19)22)32-16-7-8-17(23)18(24)9-16/h3-11H,1-2H3
MMV671933 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(O[C@H]4CN(C5CC5)[C@H]4C6=CC(F)=C(F)C=C6)N32 InChI=1S/C24H19F4N5O2/c25-17-8-3-14(9-18(17)26)22-19(12-32(22)15-4-5-15)35-21-11-29-10-20-30-31-23(33(20)21)13-1-6-16(7-2-13)34-24(27)28/h1-3,6-11,15,19,22,24H,4-5,12H2/t19-,22-/m0/s1
MMV671934 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(O[C@H]4C(N(C5CC5)[C@H]4C6=CC(F)=C(F)C=C6)=O)N32 InChI=1S/C24H17F4N5O3/c25-16-8-3-13(9-17(16)26)20-21(23(34)32(20)14-4-5-14)36-19-11-29-10-18-30-31-22(33(18)19)12-1-6-15(7-2-12)35-24(27)28/h1-3,6-11,14,20-21,24H,4-5H2/t20-,21+/m0/s1
MMV672618 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC(C=CN5C)=C5C=C4 InChI=1S/C21H15F2N5O2/c1-27-9-8-14-10-16(6-7-17(14)27)29-19-12-24-11-18-25-26-20(28(18)19)13-2-4-15(5-3-13)30-21(22)23/h2-12,21H,1H3
MMV672619 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(O[C@@H]4C(N(C5CC5)[C@@H]4C6=CC(F)=C(F)C=C6)=O)N=C3 InChI=1S/C24H17F4N5O3/c25-16-8-3-13(9-17(16)26)20-21(23(34)33(20)14-4-5-14)36-19-11-32-18(10-29-19)30-31-22(32)12-1-6-15(7-2-12)35-24(27)28/h1-3,6-11,14,20-21,24H,4-5H2/t20-,21+/m1/s1
MMV672620 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C=CC(C(F)(F)F)=N5)C5=C4 InChI=1S/C22H12F5N5O2/c23-21(24)34-14-5-2-13(3-6-14)20-31-30-18-10-28-11-19(32(18)20)33-15-7-1-12-4-8-17(22(25,26)27)29-16(12)9-15/h1-11,21H
MMV672621 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C(C)=NO5)C5=C4 InChI=1S/C20H13F2N5O3/c1-11-15-7-6-14(8-16(15)30-26-11)28-18-10-23-9-17-24-25-19(27(17)18)12-2-4-13(5-3-12)29-20(21)22/h2-10,20H,1H3
MMV672622 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C(C)=CC(C)(C)O5)C5=C4 InChI=1S/C24H20F2N4O3/c1-14-11-24(2,3)33-19-10-17(8-9-18(14)19)31-21-13-27-12-20-28-29-22(30(20)21)15-4-6-16(7-5-15)32-23(25)26/h4-13,23H,1-3H3
MMV672623 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C=NN5C)C5=C4 InChI=1S/C20H14F2N6O2/c1-27-16-8-15(7-4-13(16)9-24-27)29-18-11-23-10-17-25-26-19(28(17)18)12-2-5-14(6-3-12)30-20(21)22/h2-11,20H,1H3
MMV672624 ClC(C=C1)=CC2=C1C(CC2)NC(C3=CN=CC4=NN=C(C5=CC=C(OC(F)F)C=C5)N43)=O InChI=1S/C22H16ClF2N5O2/c23-14-4-7-16-13(9-14)3-8-17(16)27-21(31)18-10-26-11-19-28-29-20(30(18)19)12-1-5-15(6-2-12)32-22(24)25/h1-2,4-7,9-11,17,22H,3,8H2,(H,27,31)
MMV672625 CC(N(C1=CC=C(F)C=C1)N=C2C)=C2C3=NN=C4C=NC=C(C(NC5=CC(C(F)(F)F)=NC=C5)=O)N43 InChI=1S/C23H16F4N8O/c1-12-20(13(2)35(33-12)16-5-3-14(24)4-6-16)21-32-31-19-11-28-10-17(34(19)21)22(36)30-15-7-8-29-18(9-15)23(25,26)27/h3-11H,1-2H3,(H,29,30,36)
MMV672626 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC(C(F)(F)F)C4(CC4)C5=CC=C(F)C(F)=C5 InChI=1S/C23H15F7N4O2/c24-15-6-3-13(9-16(15)25)22(7-8-22)20(23(28,29)30)36-18-11-31-10-17-32-33-19(34(17)18)12-1-4-14(5-2-12)35-21(26)27/h1-6,9-11,20-21H,7-8H2
MMV672686 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC5=NN(C)C=C5C=C4 InChI=1S/C20H14F2N6O2/c1-27-11-13-4-7-15(8-16(13)26-27)29-18-10-23-9-17-24-25-19(28(17)18)12-2-5-14(6-3-12)30-20(21)22/h2-11,20H,1H3
MMV672687 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC(O)C4=CC=C(F)C(F)=C4 InChI=1S/C20H14F4N4O3/c21-14-6-3-12(7-15(14)22)16(29)10-30-18-9-25-8-17-26-27-19(28(17)18)11-1-4-13(5-2-11)31-20(23)24/h1-9,16,20,29H,10H2
MMV672688 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC4=CC=CC5=C4C=CC=C5 InChI=1S/C23H16F2N4O2/c24-23(25)31-18-10-8-16(9-11-18)22-28-27-20-12-26-13-21(29(20)22)30-14-17-6-3-5-15-4-1-2-7-19(15)17/h1-13,23H,14H2
MMV672689 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=NC=C(O[C@H]4CN(C5CC5)[C@@H]4C6=CC(F)=C(F)C=C6)N32 InChI=1S/C24H19F4N5O2/c25-17-8-3-14(9-18(17)26)22-19(12-32(22)15-4-5-15)35-21-11-29-10-20-30-31-23(33(20)21)13-1-6-16(7-2-13)34-24(27)28/h1-3,6-11,15,19,22,24H,4-5,12H2/t19-,22+/m0/s1
MMV672723 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC(O)(C)C4=CC=C(F)C(F)=C4 InChI=1S/C21H16F4N4O3/c1-21(30,13-4-7-15(22)16(23)8-13)11-31-18-10-26-9-17-27-28-19(29(17)18)12-2-5-14(6-3-12)32-20(24)25/h2-10,20,30H,11H2,1H3
MMV672725 (mentioned in Jo Ubels Ether scheme)
MMV672726 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC4CCC5=C4C=CC(Cl)=C5 InChI=1S/C22H17ClF2N4O2/c23-16-5-8-18-14(9-16)1-2-15(18)12-30-20-11-26-10-19-27-28-21(29(19)20)13-3-6-17(7-4-13)31-22(24)25/h3-11,15,22H,1-2,12H2
MMV672727 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC(F)(C)C4=CC=C(F)C(F)=C4 InChI=1S/C21H15F5N4O2/c1-21(26,13-4-7-15(22)16(23)8-13)11-31-18-10-27-9-17-28-29-19(30(17)18)12-2-5-14(6-3-12)32-20(24)25/h2-10,20H,11H2,1H3
MMV672730 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(N=C(C(F)(F)F)N=C5)C5=C4 InChI=1S/C21H11F5N6O2/c22-20(23)34-13-3-1-11(2-4-13)18-31-30-16-9-27-10-17(32(16)18)33-14-5-6-15-12(7-14)8-28-19(29-15)21(24,25)26/h1-10,20H
MMV672936 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OCC(F)C4=CC=C(F)C(F)=C4 InChI=1S/C20H13F5N4O2/c21-14-6-3-12(7-15(14)22)16(23)10-30-18-9-26-8-17-27-28-19(29(17)18)11-1-4-13(5-2-11)31-20(24)25/h1-9,16,20H,10H2
MMV672937 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C=CS5)C5=C4 InChI=1S/C20H12F2N4O2S/c21-20(22)28-14-4-2-13(3-5-14)19-25-24-17-10-23-11-18(26(17)19)27-15-6-1-12-7-8-29-16(12)9-15/h1-11,20H
MMV672939 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=CC(OCCC4=CC(F)=C(F)C=C4)=NN32 InChI=1S/C20H14F4N4O2/c21-15-6-1-12(11-16(15)22)9-10-29-18-8-7-17-25-26-19(28(17)27-18)13-2-4-14(5-3-13)30-20(23)24/h1-8,11,20H,9-10H2
MMV672940 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C=C(F)C(F)=C5)C5=C4 InChI=1S/C22H12F4N4O2/c23-17-8-13-3-6-16(7-14(13)9-18(17)24)31-20-11-27-10-19-28-29-21(30(19)20)12-1-4-15(5-2-12)32-22(25)26/h1-11,22H
MMV672941 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C(F)=CC(F)=C5)C5=C4 InChI=1S/C22H12F4N4O2/c23-14-7-13-8-16(5-6-17(13)18(24)9-14)31-20-11-27-10-19-28-29-21(30(19)20)12-1-3-15(4-2-12)32-22(25)26/h1-11,22H
MMV672942 FC(F)OC(C=C1)=CC=C1C2=NN=C3C=CC(NCCC4=CC=C(F)C=C4)=NN32 InChI=1S/C20H16F3N5O/c21-15-5-1-13(2-6-15)11-12-24-17-9-10-18-25-26-19(28(18)27-17)14-3-7-16(8-4-14)29-20(22)23/h1-10,20H,11-12H2,(H,24,27)
MMV672989 FC(F)OC(C=C1)=CC=C1C2=NN=C(N32)C=NC=C3OC4=CC=C(C=CC(OC)=N5)C5=C4 InChI=1S/C22H15F2N5O3/c1-30-19-9-5-13-2-8-16(10-17(13)26-19)31-20-12-25-11-18-27-28-21(29(18)20)14-3-6-15(7-4-14)32-22(23)24/h2-12,22H,1H3
MMV672990 FC(F)OC(C=C1)=CC=C1C2=NN=C3N2C=C(NC(CC4=CC=CC(Cl)=C4)=O)N=C3 InChI=1S/C20H14ClF2N5O2/c21-14-3-1-2-12(8-14)9-18(29)25-16-11-28-17(10-24-16)26-27-19(28)13-4-6-15(7-5-13)30-20(22)23/h1-8,10-11,20H,9H2,(H,25,29)
MMV672992 N#CC(C=C1)=CC=C1C2=NN=C3C=CC(NCCC4=CN=CC=C4)=NN32 InChI=1S/C19H15N7/c20-12-14-3-5-16(6-4-14)19-24-23-18-8-7-17(25-26(18)19)22-11-9-15-2-1-10-21-13-15/h1-8,10,13H,9,11H2,(H,22,25)
MMV675718 (OSM-S-201)
MMV675719 (OSM-S-206)
MMV675946 (OSM-S-204)
MMV675947 (OSM-S-254)
MMV675948 (OSM-S-265)
MMV675949 (OSM-S-258)
MMV675950 (OSM-S-255)
MMV675951 (OSM-S-256)
MMV675952 (OSM-S-257)
MMV675959 (OSM-S-259)
MMV675960 (OSM-S-260)
MMV675961 (OSM-S-262)
MMV675962 (OSM-S-263)
MMV675963 (OSM-S-271)
(OSM-S-187)
(OSM-S-188)
(OSM-S-189)
(OSM-S-190)
(OSM-S-191)
(OSM-S-207)
(OSM-S-208)
(OSM-S-219)
(OSM-S-220)
temp
