OpenSourceMalaria:GSK Arylpyrrole Series: Difference between revisions
Matthew Todd (talk | contribs) (→Round 3: added links to Round 3 Aery results) |
Matthew Todd (talk | contribs) (→Round 3: G+ commercial link) |
||
| (4 intermediate revisions by the same user not shown) | |||
| Line 19: | Line 19: | ||
Picture of initial second round evaluation goes here. | Picture of initial second round evaluation goes here. | ||
[https://plus.google.com/114702323662314783325/posts/Pma1Ddk2XCy Discussion of the results, and associated planning]. | |||
Though compounds with related substructures had been found to be false-positive pan-assay interference compounds (PAINS), [http://www.thesynapticleap.org/node/367 it was thought unlikely] that the present compounds suffered from this. | Though compounds with related substructures had been found to be false-positive pan-assay interference compounds (PAINS), [http://www.thesynapticleap.org/node/367 it was thought unlikely] that the present compounds suffered from this. | ||
| Line 38: | Line 38: | ||
A decision was taken to carry out a third round of analog synthesis and evaluation on the arylpyrrole series, with an emphasis on analogs a) with low logP and b) that lack the thiazolidinone heterocycle. It was decided for the moment to park the near neighbour thiazolidinones because despite potency and activity in the late stage gametocyte assay, the series [http://www.thesynapticleap.org/node/384 suffered from low solubility]. Should this set of compounds be re-started, an [http://www.thesynapticleap.org/node/400 automatic prediction of isosteric replacements] has already been done ([http://malaria.ourexperiment.org/in_silico_prediction/2954 data]). | A decision was taken to carry out a third round of analog synthesis and evaluation on the arylpyrrole series, with an emphasis on analogs a) with low logP and b) that lack the thiazolidinone heterocycle. It was decided for the moment to park the near neighbour thiazolidinones because despite potency and activity in the late stage gametocyte assay, the series [http://www.thesynapticleap.org/node/384 suffered from low solubility]. Should this set of compounds be re-started, an [http://www.thesynapticleap.org/node/400 automatic prediction of isosteric replacements] has already been done ([http://malaria.ourexperiment.org/in_silico_prediction/2954 data]). | ||
A consultation was [http://www.thesynapticleap.org/node/409 started] and [http://www.thesynapticleap.org/node/412 occurred] asking for suggestions for the ten most appropriate compounds to make, and the ten most interesting for commercial procurement. Assistance came from [http://www.thesynapticleap.org/node/399 automatic searching of databases of commercial compounds coupled with similarity searching] based on the hit compounds or other sources. The final stage of the consultation took place [http://www.youtube.com/watch?v=ooM8kuo14Bg live online]. The lists of compounds were [http://www.thesynapticleap.org/node/416 finalised] and [http://www.thesynapticleap.org/comment/863#comment-863 confirmation was secured from GSK] that none of the proposed structures had been included in the original GSK screen; commercial compounds were ordered and synthesis commenced. In late October 2012 [http://malaria.ourexperiment.org/biological_data/5010 the first biological data] were received from the Avery lab. In early November 2012, the team received results from the second phase of [http://www.thesynapticleap.org/node/430 biological evaluation] of the commercial compounds (having codes OSM-S-81 through 91) and the first synthetic compounds that had been completed ([http://malaria.ourexperiment.org/biological_data/5521 data]). This set of compounds were found to possess low to negligible levels of activity. Though surprising, the date provided some interesting insights. For example, a forked series, the [http://openwetware.org/wiki/OSDDMalaria:Arylpyrazole_Series pyrazoles], looked attractive but so far all examples tested (e.g., OSM-S-92) were found to be inactive ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/6JWcy32oAfx pictorial comparison]). It was clear that alteration of the portion of the molecule attached to the pyrrole tended to eliminate activity ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/FwVznMNSRRB picture of this] and [https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/MXnCqf4g9zx summary of the shortened analogs]). While not completely forbidden, replacement of the ester with amine or amide functionality was generally deleterious ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/ei5duHLWnAL picture of this] and [https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/5NfUwhvdRud direct amide/ester comparison]). Methylation of the terminal amide, or of the amine/amide replacing the ester, or adjacent to the ester also greatly reduces activity ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/XhfXZJagjyz picture of this]). | A consultation was [http://www.thesynapticleap.org/node/409 started] and [http://www.thesynapticleap.org/node/412 occurred] asking for suggestions for the ten most appropriate compounds to make, and the ten most interesting for commercial procurement. Assistance came from [http://www.thesynapticleap.org/node/399 automatic searching of databases of commercial compounds coupled with similarity searching] based on the hit compounds or other sources (see also [https://plus.google.com/114702323662314783325/posts/Pma1Ddk2XCy this discussion]). The final stage of the consultation took place [http://www.youtube.com/watch?v=ooM8kuo14Bg live online]. The lists of compounds were [http://www.thesynapticleap.org/node/416 finalised] and [http://www.thesynapticleap.org/comment/863#comment-863 confirmation was secured from GSK] that none of the proposed structures had been included in the original GSK screen; commercial compounds were ordered and synthesis commenced. In late October 2012 [http://malaria.ourexperiment.org/biological_data/5010 the first biological data] were received from the Avery lab. In early November 2012, the team received results from the second phase of [http://www.thesynapticleap.org/node/430 biological evaluation] of the commercial compounds (having codes OSM-S-81 through 91) and the first synthetic compounds that had been completed ([http://malaria.ourexperiment.org/biological_data/5521 data]). This set of compounds were found to possess low to negligible levels of activity. Though surprising, the date provided some interesting insights. For example, a forked series, the [http://openwetware.org/wiki/OSDDMalaria:Arylpyrazole_Series pyrazoles], looked attractive but so far all examples tested (e.g., OSM-S-92) were found to be inactive ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/6JWcy32oAfx pictorial comparison]). It was clear that alteration of the portion of the molecule attached to the pyrrole tended to eliminate activity ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/FwVznMNSRRB picture of this] and [https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/MXnCqf4g9zx summary of the shortened analogs]). While not completely forbidden, replacement of the ester with amine or amide functionality was generally deleterious ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/ei5duHLWnAL picture of this] and [https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/5NfUwhvdRud direct amide/ester comparison]). Methylation of the terminal amide, or of the amine/amide replacing the ester, or adjacent to the ester also greatly reduces activity ([https://plus.google.com/u/0/b/114702323662314783325/114702323662314783325/posts/XhfXZJagjyz picture of this]). | ||
The next batch of third round compounds were synthesised and evaluated in December 2012. As with the first batch, all of the compounds were essentially inactive (OSM-S-103 showed mild activity). [http://www.thesynapticleap.org/node/435 Summary of these data]. | The next batch of third round compounds were synthesised and evaluated in December 2012. As with the first batch, all of the compounds were essentially inactive (OSM-S-103 showed mild activity). [http://malaria.ourexperiment.org/biological_data/5981 Data] [http://www.thesynapticleap.org/node/435 Summary of all these third-round data]. | ||
[[Image:tssfar.png|thumb|center|700px| '''Representative Compounds from the Third Round]] | [[Image:tssfar.png|thumb|center|700px| '''Representative Compounds from the Third Round]] | ||
| Line 95: | Line 95: | ||
[[Image:Series 1 Synthesis Summery for wiki - prodrugs.png|thumb|center|300px| '''Pyrrole Fragments Synthesised (OSM-S- 2-4, 12-14, 28-32 were biologically evaluated)''']] | [[Image:Series 1 Synthesis Summery for wiki - prodrugs.png|thumb|center|300px| '''Pyrrole Fragments Synthesised (OSM-S- 2-4, 12-14, 28-32 were biologically evaluated)''']] | ||
Several amide analogs were made or purchased. Attempts to synthesize [http://malaria.ourexperiment.org/osm_procedures/7073/Preparation_of_OSMS19.html OSM-S-19] directly through coupling of [http://malaria.ourexperiment.org/osm_procedures/7051/Preparation_of_OSMS4.html OSM-S-4] with glycinamide using T3P or DCC failed, but the compound could be obtained through SOCl<sub>2</sub>-mediated pre-formation of the acid chloride. This approach was also employed successfully for [http://malaria.ourexperiment.org/osm_procedures/7140/Preparation_of_OSMS59.html OSM-S-59], [http://malaria.ourexperiment.org/osm_procedures/7078/Preparation_of_OSMS21.html OSM-S-21], [http://malaria.ourexperiment.org/osm_procedures/7079/Preparation_of_OSMS22.html OSM-S-22] and [http://malaria.ourexperiment.org/osm_procedures/7142/Preparation_of_OSMS61.html OSM-S-61]. [http://malaria.ourexperiment.org/osm_procedures/7175/Preparation_of_OSMS93.html OSM-S-93] was obtained by a direct coupling of the acid [http://malaria.ourexperiment.org/osm_procedures/7051/Preparation_of_OSMS4.html OSM-S-4] with methyl amine using EDCl.HCl and HOBt, a procedure that was also successfully employed with [http://malaria.ourexperiment.org/osm_procedures/7142/Preparation_of_OSMS61.html OSM-S-61]. [http://malaria.ourexperiment.org/osm_procedures/7070/Preparation_of_OSMS16.html OSM-S-16] was obtained through a T3P-mediated coupling between hippuric acid and 4-aminoantipyrene; this coupling strategy also successfully gave [http://malaria.ourexperiment.org/osm_procedures/7060/Preparation_of_OSMS8.html OSM-S-8]. The urea compound [http://malaria.ourexperiment.org/osm_procedures/7059/Preparation_of_OSMS7.html OSM-S-7] arose as a byproduct from DCC-mediated attempts at [http://malaria.ourexperiment.org/osm_procedures/7060/Preparation_of_OSMS8.html OSM-S-8] and [http://malaria.ourexperiment.org/osm_procedures/7073/Preparation_of_OSMS19.html OSM-S-19]. The amine [http://malaria.ourexperiment.org/osm_procedures/7072/Preparation_of_OSMS18.html OSM-S-18], used for the synthesis of [http://malaria.ourexperiment.org/osm_procedures/7078/Preparation_of_OSMS21.html OSM-S-21] was prepared (and used crude) by acidic deprotection of its Boc-protected precursor [http://malaria.ourexperiment.org/osm_procedures/7071/Preparation_of_OSMS17.html OSM-S-17], itself obtained from a T3P-mediated coupling of the commercially-available halves Boc-protected glycine and 4-aminoantipyrene. This route to the amine was found to be superior to an attempted saponification of [http://malaria.ourexperiment.org/osm_procedures/7070/Preparation_of_OSMS16.html OSM-S-16]. Compounds [http://malaria.ourexperiment.org/osm_procedures/7162/Preparation_of_OSMS81.html OSM-S-81], [http://malaria.ourexperiment.org/osm_procedures/7165/Preparation_of_OSMS83.html OSM-S-83], [http://malaria.ourexperiment.org/osm_procedures/7166/Preparation_of_OSMS84.html OSM-S-84], [http://malaria.ourexperiment.org/osm_procedures/7168/Preparation_of_OSMS86.html OSM-S-86] and [http://malaria.ourexperiment.org/osm_procedures/7169/Preparation_of_OSMS87.html OSM-S-87] were purchased. | Several amide analogs were made or purchased. Attempts to synthesize [http://malaria.ourexperiment.org/osm_procedures/7073/Preparation_of_OSMS19.html OSM-S-19] directly through coupling of [http://malaria.ourexperiment.org/osm_procedures/7051/Preparation_of_OSMS4.html OSM-S-4] with glycinamide using T3P or DCC failed, but the compound could be obtained through SOCl<sub>2</sub>-mediated pre-formation of the acid chloride. This approach was also employed successfully for [http://malaria.ourexperiment.org/osm_procedures/7140/Preparation_of_OSMS59.html OSM-S-59], [http://malaria.ourexperiment.org/osm_procedures/7078/Preparation_of_OSMS21.html OSM-S-21], [http://malaria.ourexperiment.org/osm_procedures/7079/Preparation_of_OSMS22.html OSM-S-22] (though this compound was not adequately characterised and not biologically evaluated) and [http://malaria.ourexperiment.org/osm_procedures/7142/Preparation_of_OSMS61.html OSM-S-61]. [http://malaria.ourexperiment.org/osm_procedures/7175/Preparation_of_OSMS93.html OSM-S-93] was obtained by a direct coupling of the acid [http://malaria.ourexperiment.org/osm_procedures/7051/Preparation_of_OSMS4.html OSM-S-4] with methyl amine using EDCl.HCl and HOBt, a procedure that was also successfully employed with [http://malaria.ourexperiment.org/osm_procedures/7142/Preparation_of_OSMS61.html OSM-S-61]. [http://malaria.ourexperiment.org/osm_procedures/7070/Preparation_of_OSMS16.html OSM-S-16] was obtained through a T3P-mediated coupling between hippuric acid and 4-aminoantipyrene; this coupling strategy also successfully gave [http://malaria.ourexperiment.org/osm_procedures/7060/Preparation_of_OSMS8.html OSM-S-8]. The urea compound [http://malaria.ourexperiment.org/osm_procedures/7059/Preparation_of_OSMS7.html OSM-S-7] arose as a byproduct from DCC-mediated attempts at [http://malaria.ourexperiment.org/osm_procedures/7060/Preparation_of_OSMS8.html OSM-S-8] and [http://malaria.ourexperiment.org/osm_procedures/7073/Preparation_of_OSMS19.html OSM-S-19]. The amine [http://malaria.ourexperiment.org/osm_procedures/7072/Preparation_of_OSMS18.html OSM-S-18], used for the synthesis of [http://malaria.ourexperiment.org/osm_procedures/7078/Preparation_of_OSMS21.html OSM-S-21] was prepared (and used crude) by acidic deprotection of its Boc-protected precursor [http://malaria.ourexperiment.org/osm_procedures/7071/Preparation_of_OSMS17.html OSM-S-17], itself obtained from a T3P-mediated coupling of the commercially-available halves Boc-protected glycine and 4-aminoantipyrene. This route to the amine was found to be superior to an attempted saponification of [http://malaria.ourexperiment.org/osm_procedures/7070/Preparation_of_OSMS16.html OSM-S-16]. Compounds [http://malaria.ourexperiment.org/osm_procedures/7162/Preparation_of_OSMS81.html OSM-S-81], [http://malaria.ourexperiment.org/osm_procedures/7165/Preparation_of_OSMS83.html OSM-S-83], [http://malaria.ourexperiment.org/osm_procedures/7166/Preparation_of_OSMS84.html OSM-S-84], [http://malaria.ourexperiment.org/osm_procedures/7168/Preparation_of_OSMS86.html OSM-S-86] and [http://malaria.ourexperiment.org/osm_procedures/7169/Preparation_of_OSMS87.html OSM-S-87] were purchased. | ||
[[Image:Series 1 Synthesis Summary for wiki - amide digest.png|thumb|center|700px| '''Synthesis and Purchase of a Set of Amide Analogs of the Original Arylpyrrole hits''']] | [[Image:Series 1 Synthesis Summary for wiki - amide digest.png|thumb|center|700px| '''Synthesis and Purchase of a Set of Amide Analogs of the Original Arylpyrrole hits''']] | ||
| Line 142: | Line 142: | ||
Cc1ccnc(c1)n2c(cc(c2C)C=C3C(=O)N(C(=Nc4ccccc4)S3)Cc5ccco5)C, InChI=1S/C27H24N4O2S/c1-18-11-12-28-25(14-18)31-19(2)15-21(20(31)3)16-24-26(32)30(17-23-10-7-13-33-23)27(34-24)29-22-8-5-4-6-9-22/h4-16H,17H2,1-3H3/b24-16+,29-27+, CKMRVKNPWCFQLU-NEQGSXNKSA-N | Cc1ccnc(c1)n2c(cc(c2C)C=C3C(=O)N(C(=Nc4ccccc4)S3)Cc5ccco5)C, InChI=1S/C27H24N4O2S/c1-18-11-12-28-25(14-18)31-19(2)15-21(20(31)3)16-24-26(32)30(17-23-10-7-13-33-23)27(34-24)29-22-8-5-4-6-9-22/h4-16H,17H2,1-3H3/b24-16+,29-27+, CKMRVKNPWCFQLU-NEQGSXNKSA-N | ||
TCMDC-124103, | TCMDC-124103, CHEMBL597849, CHEMBL: [https://www.ebi.ac.uk/chembl/compound/inspect/CHEMBL597849 597849] | ||
Cc1cc(cc(c1)n2c(cc(c2C)C=C3C(=O)NC(=Nc4ccc(cc4)Cl)S3)C)C, InChI=1S/C24H22ClN3OS/c1-14-9-15(2)11-21(10-14)28-16(3)12-18(17(28)4)13-22-23(29)27-24(30-22)26-20-7-5-19(25)6-8-20/h5-13H,1-4H3,(H,26,27,29)/b22-13+, WVSGKKHBDQIDEU-LPYMAVHISA-N | Cc1cc(cc(c1)n2c(cc(c2C)C=C3C(=O)NC(=Nc4ccc(cc4)Cl)S3)C)C, InChI=1S/C24H22ClN3OS/c1-14-9-15(2)11-21(10-14)28-16(3)12-18(17(28)4)13-22-23(29)27-24(30-22)26-20-7-5-19(25)6-8-20/h5-13H,1-4H3,(H,26,27,29)/b22-13+, WVSGKKHBDQIDEU-LPYMAVHISA-N | ||
Revision as of 08:39, 15 August 2014
This page is about the first series of compounds investigated in the Open Source Malaria (OSM) project. See elsewhere for general information on OSM, the Story so Far, or other series.
The Arylpyrrole Series
Series was released in a large dataset by GSK and later listed as one of the most promising leads from the set (though lacking aryl F)
Resynthesis and Round 1
Paul Ylioja started Series 1 by resynthesising the two known active arylpyrroles from the original GSK screen (OSM-S-5 and OSM-S-6 - structures below), plus a few simple derivatives, and confirming that they were active. The biological evaluation was carried out by three separate labs (Vicky Avery, Stuart Ralph and the original GSK Tres Cantos Lab led by Javier Gamo) to ensure reproducibility. The original compounds contained an ester which was thought likely to hydrolyze in vivo, so various versions of the "lower half" of these leads were also evaluated to check whether the original hits were prodrugs, but all these compounds were found to be inactive. Paul Willis from MMV recommended a few "near neighbor" compounds that also looked interesting, and a number of these were made too and evaluated in this first round. One compound, OSM-S-9, was found to be more active than the original GSK hits. Sanjay Batra and his student Soumya made some analogs varying in the position of the fluorine atom, though the activity of those tested to date is low. The outcome from Round 1 was that the original GSK hits remained interesting (because of their reasonable potency and logP values) but that highly potent novel antimalarials were also being generated in this class within the near neighbor set.

Round 2
A second set of compounds was synthesized and evaluated, giving rise to several new highly potent compounds, one of which (OSM-S-39) displayed a picomolar IC50 value. Data were obtained from the Avery and Ralph labs and GSK.
Picture of initial second round evaluation goes here.
Discussion of the results, and associated planning.
Though compounds with related substructures had been found to be false-positive pan-assay interference compounds (PAINS), it was thought unlikely that the present compounds suffered from this.
In-depth Biological Evaluation of Most Promising Compounds from Rounds 1 and 2
It was decided to move the most promising compounds on to advanced biological evaluation, to assess the promise of this class early rather than continue to increase potency through analog synthesis:
- Metabolic and solubility assays: The two original GSK compounds (OSM-S-5 and OSM-S-6) and six other compounds made in this project were evaluated by Sue Charman's lab at Monash for their stability in phosphate buffer. The raw data are here and can be discussed here. The GSK originals displayed good solubility but moderate degradation rates. The other compounds were degraded more slowly but at a cost of low solubility. Subsequently the original GSK compound OSM-S-5 was evaluated for stability in human and mouse plasma. The compound was stable in human plasma but susceptible to hydrolysis in mouse plasma. Esterase activity is known to be higher in rodents than in other species which was confirmed using a control compound in this assay (p-nitrophenol acetate) so the results are not too surprising. Lab book page here. A glutathione trapping experiment was carried out on OSM-S-35 as representative of the Near Neighbour series, giving some identification of possible trapped metabolites, but the levels observed were not large. This mirrored experiments with an analogous compound that did not give rise to Michael addition adducts when mixed with benzylthiol.
- hERG: One of the original GSK compounds (OSM-S-5) plus one of the most potent novel compounds identified to date (OSM-S-35) were subjected to the hERG assay and passed, perhaps implying that this class of compounds should not exhibit undesirable cardiac side effects. Discussion page here.
- Late Stage Gametocyte Assay: Four of the compounds have also been evaluated in a late stage gametocyte assay with very interesting results indicating unusually high activity in blocking the transmission of the parasite. The original GSK compound OSM-S-5 was inactive. Discussion page here.
- In vivo: However, the two original GSK compounds as well as one of the most promising near neighbor compounds (OSM-S-35) were evaluated in mice and found to possess zero oral efficacy (Results available here). Subsequent analysis of the plasma samples from the trial with one of the GSK compounds (OSM-S-5) showed that the compound was indeed orally available, but levels in the blood were not being maintained. Raw data here.
Other discussions of the biological results described above can be found here. The choice became whether to change the focus of the OSM project to another compound series or whether to continue to alter the structures of the most promising compounds to overcome the in vivo roadblock described above.
Round 3
A decision was taken to carry out a third round of analog synthesis and evaluation on the arylpyrrole series, with an emphasis on analogs a) with low logP and b) that lack the thiazolidinone heterocycle. It was decided for the moment to park the near neighbour thiazolidinones because despite potency and activity in the late stage gametocyte assay, the series suffered from low solubility. Should this set of compounds be re-started, an automatic prediction of isosteric replacements has already been done (data).
A consultation was started and occurred asking for suggestions for the ten most appropriate compounds to make, and the ten most interesting for commercial procurement. Assistance came from automatic searching of databases of commercial compounds coupled with similarity searching based on the hit compounds or other sources (see also this discussion). The final stage of the consultation took place live online. The lists of compounds were finalised and confirmation was secured from GSK that none of the proposed structures had been included in the original GSK screen; commercial compounds were ordered and synthesis commenced. In late October 2012 the first biological data were received from the Avery lab. In early November 2012, the team received results from the second phase of biological evaluation of the commercial compounds (having codes OSM-S-81 through 91) and the first synthetic compounds that had been completed (data). This set of compounds were found to possess low to negligible levels of activity. Though surprising, the date provided some interesting insights. For example, a forked series, the pyrazoles, looked attractive but so far all examples tested (e.g., OSM-S-92) were found to be inactive (pictorial comparison). It was clear that alteration of the portion of the molecule attached to the pyrrole tended to eliminate activity (picture of this and summary of the shortened analogs). While not completely forbidden, replacement of the ester with amine or amide functionality was generally deleterious (picture of this and direct amide/ester comparison). Methylation of the terminal amide, or of the amine/amide replacing the ester, or adjacent to the ester also greatly reduces activity (picture of this).
The next batch of third round compounds were synthesised and evaluated in December 2012. As with the first batch, all of the compounds were essentially inactive (OSM-S-103 showed mild activity). Data Summary of all these third-round data.

At the end of 2012 it appeared that the series was producing diminishing returns but there remained a small number of compounds which needed to be made to complete the campaign - specifically isosters of the troublesome ester in the original GSK hit. Patrick Thompson, a collaborator from the University of Edinburgh built on Matin Dean's work on the sulfonamide whilst Murray Robertson and Alice Williamson focused on synthesising further analogues of the near-neighbours, trying to find potent molecules with increased solubility. The near-neighbours were evaluated and a number of potent compounds were discovered, some with lower LogP than the first set of near-neighbour compounds. OSM-S-111 was evaluated in a late stage gametocyte assay and in liver stages.
Mechanism of Action
Series 1 compound have been identified from a phenotypic screen. The mechanism of action is unknown.
Iain Wallace from ChEMBL has performed a prediction of the biological role of these compounds (as well as predictions for the whole "Malaria Box", which is a set of compounds MMV are providing to people for antimalarial screening, and for all the antimalarials in ChEMBL. Iain clustered the compounds as similarity maps, allowing visualization of the correlation between structure and predicted activity. Discussed also here. One of the predictions was that the compounds in Series 1 should hit an enzyme known as DHODH. GSK screened some of the project's compounds against this enzyme (done - data need to be added). These predictions are made using informatics - a comparison of the structures of our compounds with other known compounds that have known activities. The argument is based on extrapolation.
To evaluate whether the prediction was correct, a subset of compounds were sent to Corey Nislow at the University of Toronto for screening in a yeast-based assay that does not identify for sure what the compounds are doing (which is very difficult) but provides harder biological evidence for a role. There are occasional other clues about activity arising from studies of these, or related compounds, in the literature; in such cases it is not clear whether the activity is relevant to their antimalarial potency, or whether the compounds are highlighted in assays because they are frequently members of commercial libraries.
Incorporate:
In silico prediction of targets has been carried out by Iain Wallace and summarised by Mat on the synaptic leap. Detail on the procedure is given on the ELN. The following targets were identified from data derived from the ChEMBL database:
- Carboxy-terminal domain RNA polymerase II polypeptide A small phosphatase 1
- Dihydroorotate dehydrogenase (DHODH)[in progress at GSK Tres Cantos]
- SUMO-activating enzyme subunit 2
- SUMO-activating enzyme subunit 1
- Cyclin-dependent kinase 1
Related Series
Gilbert et al.: J. Med. Chem., 2013, 56 (7), pp 2975–2990 (malaria) Roberts et al.: Bioorg. Med. Chem. Lett. 2011, 21, 6739–6745 (GPCR)
Conclusions and Current Status
Comments needed here.
The OSM project has to date had no luck in securing donations of compounds from commercial suppliers. On considering some structurally 'similar' active compounds from the TCAMS set, the team have also decided to synthesise a few extra compounds plus hybrids, in order to assess their biological activity.
Remaining Possible Lines of Enquiry in Series 1
Though the series has been parked, anyone is free to re-investigate. Of interest might be:
- Synthesise these compounds that might arise from the cyclisation of the arylpyrrole side chain
- Evaluation of the "other half" of the original GSK hit containing the antipyrene
- A new search of the TCAMS data set revealed other compounds similar to the original hits that could be used for generating "hybrid" compounds that might represent new targets for the series
Synthetic Chemistry in this Series
(Each compound with an OSM number has a dedicated page listing all preparations of that compound and any other associated data. To find these details go to this picture list (can be slow to load) or this number/string list.)
The two GSK hits TCMDC-123812 (OSM-S-5) and TCMDC-123794 (OSM-S-6) were synthesised via the novel pyrrole acid OSM-S-4 that was prepared using a Paal-Knorr cyclization of the relevant aniline and ethyl 2-acetyl-4-oxopentanoate (procedure adapting Lit 1 and Lit 2) to give the novel pyrrole ester OSM-S-3 which could be saponified to the acid. This approach was found to be superior to an alternative involving initial synthesis of the unfunctionalised pyrrole OSM-S-1, followed by conversion to the known aldehyde OSM-S-2 with a Vilsmeier-Haack reaction (a procedure that was improved through a community suggestion) and then oxidation to OSM-S-4, because the aldehyde was found to be remarkably resilient to a range of oxidants. (An alternative route to the ester OSM-S-3 using a Friedel-Crafts acylation between the unsubstituted pyrrole OSM-S-1 and ethyl chloroformate, suggested in an email from the community, gave only starting material in two attempts). Conversion of the acid to OSM-S-5 (TCMDC-123812), the first of the original GSK hits to be resynthesised, was achieved using a base and bromoacetamide; this was found to be superior to a number of attempted alternatives including coupling with glycolamide either via the pyrrole's acid chloride or directly through the use of coupling reagents (diisopropylcarbodiimide (DIC) or propylphosphonic anhydride (T3P)) (Summary of all approaches). The second GSK hit TCMDC-123794 (OSM-S-6) however was successfully synthesised from the acid OSM-S-4 via the coupling of the corresponding acid chloride with a functionalised antipyrene moiety (OSM-S-192), itself made from commercially available 4-aminoantipyrene and glycolic acid, using the acetonide of the latter (OSM-S-193).

The "lower half" aryl pyrrole "prodrugs" were made as the aldehydes, esters and acids and some were biologically evaluated (as above) - synthetic pages may be found in the strings section below.

Several amide analogs were made or purchased. Attempts to synthesize OSM-S-19 directly through coupling of OSM-S-4 with glycinamide using T3P or DCC failed, but the compound could be obtained through SOCl2-mediated pre-formation of the acid chloride. This approach was also employed successfully for OSM-S-59, OSM-S-21, OSM-S-22 (though this compound was not adequately characterised and not biologically evaluated) and OSM-S-61. OSM-S-93 was obtained by a direct coupling of the acid OSM-S-4 with methyl amine using EDCl.HCl and HOBt, a procedure that was also successfully employed with OSM-S-61. OSM-S-16 was obtained through a T3P-mediated coupling between hippuric acid and 4-aminoantipyrene; this coupling strategy also successfully gave OSM-S-8. The urea compound OSM-S-7 arose as a byproduct from DCC-mediated attempts at OSM-S-8 and OSM-S-19. The amine OSM-S-18, used for the synthesis of OSM-S-21 was prepared (and used crude) by acidic deprotection of its Boc-protected precursor OSM-S-17, itself obtained from a T3P-mediated coupling of the commercially-available halves Boc-protected glycine and 4-aminoantipyrene. This route to the amine was found to be superior to an attempted saponification of OSM-S-16. Compounds OSM-S-81, OSM-S-83, OSM-S-84, OSM-S-86 and OSM-S-87 were purchased.

Amines
The hindered ester analogs (codes) were accessible via. The ketone analogs would be accessed with Friedel-Crafts chemistry. The ether analog could not be obtained, despite many attempts including XXX.
Scheme of hindered ester, ketone and ether analogs.
Pyrazoles, oxazoles
Sulfonamides
Near Neighbour Synthesis
Synthetic Routes to Analogs Proposed By Others
The CRO Asclepia, via Frederik Doroose, contributed these synthetic routes to the project:
sD and sI compounds: Word file. sC, sD, sF, sJ, sI: Word file. Note prices are for S/M and should not be taken as a quote value.
Data on Structures on this page
Out of Date: A number of these compounds and intermediates are available for biological testing from by request (contact information on the Landing Page). If you would like to contribute new analogs, please check the list of desired compounds.
The Near-Neighbour Sub-series
Known Near Neighbours" Contained in the Original Tres Cantos Set

Data/links for these compounds:
TCMDC-123563, CHEMBL546966, CHEMBL page: 637010 Cc1ccc(cc1)n2c(cc(c2C)C(=O)CN3C(=O)C(NC3=O)Cc4ccccc4)C, InChI=1S/C25H25N3O3/c1-16-9-11-20(12-10-16)28-17(2)13-21(18(28)3)23(29)15-27-24(30)22(26-25(27)31)14-19-7-5-4-6-8-19/h4-13,22H,14-15H2,1-3H3,(H,26,31), VABUILLAJJHODP-UHFFFAOYSA-N
TCMDC-125698, CHEMBL587989, CHEMBL: 627784 Cc1cc(c(n1c2ccc(cc2)Cl)C)C=C3C(=O)N(C(=Nc4ccccc4)S3)C5CCCC5, InChI=1S/C27H26ClN3OS/c1-18-16-20(19(2)30(18)24-14-12-21(28)13-15-24)17-25-26(32)31(23-10-6-7-11-23)27(33-25)29-22-8-4-3-5-9-22/h3-5,8-9,12-17,23H,6-7,10-11H2,1-2H3/b25-17+,29-27+, DHRLFEJASBKCIX-BDXOCSITSA-N
TCMDC-125697, CHEMBL581336, CHEMBL: 640978 CCOC(=O)c1ccc(cc1)n2c(cc(c2C)C=C3C(=O)N(C(=Nc4ccccc4)S3)C5CCCC5)C, InChI=1S/C30H31N3O3S/c1-4-36-29(35)22-14-16-26(17-15-22)32-20(2)18-23(21(32)3)19-27-28(34)33(25-12-8-9-13-25)30(37-27)31-24-10-6-5-7-11-24/h5-7,10-11,14-19,25H,4,8-9,12-13H2,1-3H3/b27-19+,31-30+, AICPZFOVJMLAFN-VDHWCBMGSA-N
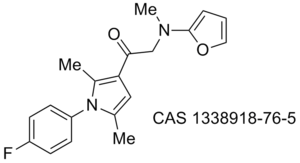
TCMDC-125659, CHEMBL528140, CHEMBL: 626220 Cc1ccnc(c1)n2c(cc(c2C)C=C3C(=O)N(C(=Nc4ccccc4)S3)Cc5ccco5)C, InChI=1S/C27H24N4O2S/c1-18-11-12-28-25(14-18)31-19(2)15-21(20(31)3)16-24-26(32)30(17-23-10-7-13-33-23)27(34-24)29-22-8-5-4-6-9-22/h4-16H,17H2,1-3H3/b24-16+,29-27+, CKMRVKNPWCFQLU-NEQGSXNKSA-N
TCMDC-124103, CHEMBL597849, CHEMBL: 597849 Cc1cc(cc(c1)n2c(cc(c2C)C=C3C(=O)NC(=Nc4ccc(cc4)Cl)S3)C)C, InChI=1S/C24H22ClN3OS/c1-14-9-15(2)11-21(10-14)28-16(3)12-18(17(28)4)13-22-23(29)27-24(30-22)26-20-7-5-19(25)6-8-20/h5-13H,1-4H3,(H,26,27,29)/b22-13+, WVSGKKHBDQIDEU-LPYMAVHISA-N
TCMDC-124456, CHEMBL548395, CHEMBL: 640006 CCn1c(cc(c1C)C=C2C(=O)NC(=Nc3ccccc3)S2)C, InChI=1S/C18H19N3OS/c1-4-21-12(2)10-14(13(21)3)11-16-17(22)20-18(23-16)19-15-8-6-5-7-9-15/h5-11H,4H2,1-3H3,(H,19,20,22)/b16-11+, ZPQHJGQIEUHJLQ-LFIBNONCSA-N
Initial synthesis strategy toward near-neighbours

Experimental information available from PMY 13-1, PMY 14-1 and PMY 16-1, PMY 14-3.
Current synthesis strategy toward near-neighbours

Synthesis method taken from this paper.
Alternative side-chains
Some alternative heterocyclic side-chain ideas have been proposed here at the Synaptic Leap.
Oxazoles
The [1] side chain would target ester isosteres (see TCMDC-123812) but with greater biological stability.
- Synthesis: oxazole synthesis
- Pyrrole core acid to amide: Amide Synthesis
Other Known Incidences of Molecules in this Series
According to this paper, similar compounds are agonists of the sphingosine-1-phosphate receptor subtypes 1-5, which have a role in immune system function.
A new class of antimalarial was recently published by Novartis, Imidazolopiperazines.
Misc papers to digest/assimilate regarding antimycobacterial/tuberculosis activity: Antimycobacterial 1,5-diphenyl pyrroles, 10.1016/j.ejmech.2009.06.005, 10.1016/j.bmc.2010.09.006, 10.1002/cmdc.201000526
ChEMBL-NTD
Searches on ChEMBL-NTD reveal:
- 19 iminothiazolidinones
- 55 2,5-dialkylpyrroles
- 58 rhodanines. These do not contain any biological data, presumably omitted due to promiscuity/PAINS issues.
Scifinder Search: 2,4,5-alkyl-1-aryl-pyrrole: 77552 hits, 905 biological studies:
Obesity/diabetes GIP receptor inhibitor JP 2011184298 (A)

Related compounds are known to inhibit the proteasome, according to this Nature paper. There is a related set of slides from a company, Progenra. The lead compound (IU1, CAS 314245-33-5) is commercially available. Related patent WO 2011094545 (A2)

WO2006076202 Steroid nuclear receptor ligands
WO 2011075684 (A1) Inhibitors of Plasma Kallikrein/Protease
WO 2009137133 (A2) 5-Substituted-2-Imino-Thiazolidinone Compounds And Their Use As Inhibitors Of Bacterial Infection
Strings and Data on Compounds on this Page
There are picture- and strings- based lists of all OSM compounds. Picture files on this wiki page (and original Chemdraw files) may all be found here. Strings are additionally listed below for the benefit of search engines.
OSM-S-1 FC1=CC=C(N2C(C)=CC=C2C)C=C1 InChI=1S/C12H12FN/c1-9-3-4-10(2)14(9)12-7-5-11(13)6-8-12/h3-8H,1-2H3
OSM-S-2 CC(N1C2=CC=C(F)C=C2)=C(C=O)C=C1C InChI=1S/C13H12FNO/c1-9-7-11(8-16)10(2)15(9)13-5-3-12(14)4-6-13/h3-8H,1-2H3
OSM-S-3 O=C(C1=C(C)N(C2=CC=C(F)C=C2)C(C)=C1)OCC InChI=1S/C15H16FNO2/c1-4-19-15(18)14-9-10(2)17(11(14)3)13-7-5-12(16)6-8-13/h5-9H,4H2,1-3H3
OSM-S-4 CC(N1C2=CC=C(F)C=C2)=C(C(O)=O)C=C1C InChI=1S/C13H12FNO2/c1-8-7-12(13(16)17)9(2)15(8)11-5-3-10(14)4-6-11/h3-7H,1-2H3,(H,16,17)
OSM-S-5 CC(N1C2=CC=C(F)C=C2)=C(C(OCC(N)=O)=O)C=C1C InChI=1S/C15H15FN2O3/c1-9-7-13(15(20)21-8-14(17)19)10(2)18(9)12-5-3-11(16)4-6-12/h3-7H,8H2,1-2H3,(H2,17,19)
OSM-S-6 CC(N1C2=CC=C(F)C=C2)=CC(C(OCC(NC(C3=O)=C(N(N3C4=CC=CC=C4)C)C)=O)=O)=C1C InChI=1S/C26H25FN4O4/c1-16-14-22(17(2)30(16)20-12-10-19(27)11-13-20)26(34)35-15-23(32)28-24-18(3)29(4)31(25(24)33)21-8-6-5-7-9-21/h5-14H,15H2,1-4H3,(H,28,32)
OSM-S-7 FC1=CC=C(N2C(C)=CC(C(N(C(NC3CCCCC3)=O)C4CCCCC4)=O)=C2C)C=C1 InChI=1S/C26H34FN3O2/c1-18-17-24(19(2)29(18)23-15-13-20(27)14-16-23)25(31)30(22-11-7-4-8-12-22)26(32)28-21-9-5-3-6-10-21/h13-17,21-22H,3-12H2,1-2H3,(H,28,32)
OSM-S-8 FC1=CC=C(N2C(C)=CC(C(N([H])C3=C(C)N(C)N(C4=CC=CC=C4)C3=O)=O)=C2C)C=C1
FC1=CC=C(N2C(C)=CC(C(NC3=C(C)N(C)N(C4=CC=CC=C4)C3=O)=O)=C2C)C=C1 InChI=1S/C24H23FN4O2/c1-15-14-21(16(2)28(15)19-12-10-18(25)11-13-19)23(30)26-22-17(3)27(4)29(24(22)31)20-8-6-5-7-9-20/h5-14H,1-4H3,(H,26,30)
OSM-S-12 CC(N1C2=CC=CC=C2)=CC(C(O)=O)=C1C InChI=1S/C13H13NO2/c1-9-8-12(13(15)16)10(2)14(9)11-6-4-3-5-7-11/h3-8H,1-2H3,(H,15,16)
OSM-S-13 CC1=CC=C(N2C(C)=C(C(O)=O)C=C2C)C=C1 InChI=1S/C14H15NO2/c1-9-4-6-12(7-5-9)15-10(2)8-13(11(15)3)14(16)17/h4-8H,1-3H3,(H,16,17)
OSM-S-14 CC(N1C2=CC=C(C(F)(F)F)C=C2)=CC(C(O)=O)=C1C InChI=1S/C14H12F3NO2/c1-8-7-12(13(19)20)9(2)18(8)11-5-3-10(4-6-11)14(15,16)17/h3-7H,1-2H3,(H,19,20)
OSM-S-16 O=C(C1=CC=CC=C1)N([H])CC(N([H])C2=C(C)N(C)N(C3=CC=CC=C3)C2=O)=O InChI=1S/C20H20N4O3/c1-14-18(20(27)24(23(14)2)16-11-7-4-8-12-16)22-17(25)13-21-19(26)15-9-5-3-6-10-15/h3-12H,13H2,1-2H3,(H,21,26)(H,22,25)
OSM-S-19 FC1=CC=C(N2C(C)=CC(C(N([H])CC(N)=O)=O)=C2C)C=C1 InChI=1S/C15H16FN3O2/c1-9-7-13(15(21)18-8-14(17)20)10(2)19(9)12-5-3-11(16)4-6-12/h3-7H,8H2,1-2H3,(H2,17,20)(H,18,21)
OSM-S-21 FC1=CC=C(N2C(C)=CC(C(N([H])CC(N([H])C3=C(C)N(C)N(C4=CC=CC=C4)C3=O)=O)=O)=C2C)C=C1 InChI=1S/C26H26FN5O3/c1-16-14-22(17(2)31(16)20-12-10-19(27)11-13-20)25(34)28-15-23(33)29-24-18(3)30(4)32(26(24)35)21-8-6-5-7-9-21/h5-14H,15H2,1-4H3,(H,28,34)(H,29,33)
OSM-S-22 FC1=CC=C(N2C(C)=CC(C(N)=O)=C2C)C=C1 InChI=1S/C13H13FN2O/c1-8-7-12(13(15)17)9(2)16(8)11-5-3-10(14)4-6-11/h3-7H,1-2H3,(H2,15,17)
OSM-S-25 CC1=CC=C(C)N1C2=CC=CC=C2 InChI=1S/C12H13N/c1-10-8-9-11(2)13(10)12-6-4-3-5-7-12/h3-9H,1-2H3
OSM-S-26 CC1=CC=C(C)N1C2=CC=C(C)C=C2 InChI=1S/C13H15N/c1-10-4-8-13(9-5-10)14-11(2)6-7-12(14)3/h4-9H,1-3H3
OSM-S-27 CC1=CC=C(C)N1C2=CC=C(C(F)(F)F)C=C2 InChI=1S/C13H12F3N/c1-9-3-4-10(2)17(9)12-7-5-11(6-8-12)13(14,15)16/h3-8H,1-2H3
OSM-S-28 CC(N1C2=CC=CC=C2)=CC(C=O)=C1C InChI=1S/C13H13NO/c1-10-8-12(9-15)11(2)14(10)13-6-4-3-5-7-13/h3-9H,1-2H3
OSM-S-29 CC1=CC=C(N2C(C)=C(C=O)C=C2C)C=C1 InChI=1S/C14H15NO/c1-10-4-6-14(7-5-10)15-11(2)8-13(9-16)12(15)3/h4-9H,1-3H3
OSM-S-30 CCOC(C1=C(C)N(C2=CC=C(C=C2)C)C(C)=C1)=O InChI=1S/C16H19NO2/c1-5-19-16(18)15-10-12(3)17(13(15)4)14-8-6-11(2)7-9-14/h6-10H,5H2,1-4H3
OSM-S-31 O=C(C1=C(C)N(C2=CC=CC=C2)C(C)=C1)OCC InChI=1S/C15H17NO2/c1-4-18-15(17)14-10-11(2)16(12(14)3)13-8-6-5-7-9-13/h5-10H,4H2,1-3H3
OSM-S-32 O=C(C1=C(C)N(C2=CC=C(C(F)(F)F)C=C2)C(C)=C1)OCC InChI=1S/C16H16F3NO2/c1-4-22-15(21)14-9-10(2)20(11(14)3)13-7-5-12(6-8-13)16(17,18)19/h5-9H,4H2,1-3H3
OSM-S-34 CC1=C(C=O)C=C(C)N1C2=CC=C(C(F)(F)F)C=C2 InChI=1S/C14H12F3NO/c1-9-7-11(8-19)10(2)18(9)13-5-3-12(4-6-13)14(15,16)17/h3-8H,1-2H3
OSM-S-59 FC1=CC=C(N2C(C)=CC(C(N(C)CC(N)=O)=O)=C2C)C=C1 InChI=1S/C16H18FN3O2/c1-10-8-14(16(22)19(3)9-15(18)21)11(2)20(10)13-6-4-12(17)5-7-13/h4-8H,9H2,1-3H3,(H2,18,21)
OSM-S-61 FC1=CC=C(N2C(C)=CC(C(N([H])C(CO)C(OC)=O)=O)=C2C)C=C1 InChI=1S/C17H19FN2O4/c1-10-8-14(16(22)19-15(9-21)17(23)24-3)11(2)20(10)13-6-4-12(18)5-7-13/h4-8,15,21H,9H2,1-3H3,(H,19,22)
OSM-S-81 CC1=CC(C(N([H])CC(N(C)C)=O)=O)=C(C)N1C2=CC=CC=C2 InChI=1S/C17H21N3O2/c1-12-10-15(17(22)18-11-16(21)19(3)4)13(2)20(12)14-8-6-5-7-9-14/h5-10H,11H2,1-4H3,(H,18,22)
OSM-S-83 CC1=CC(C(N2CC(NCC2)=O)=O)=C(C)N1C3=CC=CC=C3 InChI=1S/C17H19N3O2/c1-12-10-15(17(22)19-9-8-18-16(21)11-19)13(2)20(12)14-6-4-3-5-7-14/h3-7,10H,8-9,11H2,1-2H3,(H,18,21)
OSM-S-84 CC1=CC(C(N(C)CC(N([H])C)=O)=O)=C(C)N1C2=CC=C(Br)C=C2 InChI=1S/C17H20BrN3O2/c1-11-9-15(17(23)20(4)10-16(22)19-3)12(2)21(11)14-7-5-13(18)6-8-14/h5-9H,10H2,1-4H3,(H,19,22)
OSM-S-86 FC1=CC=C(N2C(C)=CC(C(N(CC)CC(N([H])C3=C(F)C=CC=C3F)=O)=O)=C2C)C=C1 InChI=1S/C23H22F3N3O2/c1-4-28(13-21(30)27-22-19(25)6-5-7-20(22)26)23(31)18-12-14(2)29(15(18)3)17-10-8-16(24)9-11-17/h5-12H,4,13H2,1-3H3,(H,27,30)
OSM-S-87 CC1=CC(C(N(C)CC(N([H])C2=NOC(C)=C2)=O)=O)=C(C)N1C3=CC(C)=CC=C3 InChI=1S/C21H24N4O3/c1-13-7-6-8-17(9-13)25-14(2)10-18(16(25)4)21(27)24(5)12-20(26)22-19-11-15(3)28-23-19/h6-11H,12H2,1-5H3,(H,22,23,26)
OSM-S-93 FC1=CC=C(N2C(C)=CC(C(N(C)C)=O)=C2C)C=C1 InChI=1S/C15H17FN2O/c1-10-9-14(15(19)17(3)4)11(2)18(10)13-7-5-12(16)6-8-13/h5-9H,1-4H3
OSM-S-192 OCC(NC1=C(C)N(C)N(C2=CC=CC=C2)C1=O)=O InChI=1S/C13H15N3O3/c1-9-12(14-11(18)8-17)13(19)16(15(9)2)10-6-4-3-5-7-10/h3-7,17H,8H2,1-2H3,(H,14,18)
OSM-S-193 O=C1COC(C)(C)O1 InChI=1S/C5H8O3/c1-5(2)7-3-4(6)8-5/h3H2,1-2H3
