Lidstrom:QuikChange Site-Directed Mutagenesis
From OpenWetWare
Back to Protocols
Manuals
- QuikChange II: use 2 oligos (reverse compliments) and only do mutations at one priming site
- QuikChange II Lightning: use 1 oligo, and can introduce mutations at multiple sites
QuikChange Basics
- You can buy a kit from Agilent or use your own ingredients.
- The marketing term "lightning" refers to an improved Dpn1, that degrades template (unmutated template) more efficiently.
- If you buy the kits, 10uL rxns are plenty (this is ~4x less than the protocol suggests) and transformations using 10uL of competent cells are suitable. If you are introducing mutations with multiple priming sites, you could consider transforming more.
Quik Solution
- Quik Solution is probably pure DMSO.
Two (main) QuikChange kits are available
- QuikChange Lightning.
- For mutations contained within a single primer
- QuikChange Lightning Multi
- For mutations contained in >1 primer
- Can be used for mutations contained in 1 primer, but this kit is more expensive than the single kit and likely has lower efficiency.
- Both kits:
- come with chemically competent XL10-Gold. This is perhaps the most valuable part of the kit.
Primer Design
- Use online primer design tool. Account required.
- Use guidelines in the manual:

primer design instructions from the manual
Reaction Recipes
- Regular (2 reverse compliment primers)
- Lightning:

reaction recipe instructions from the Lightning manual - QuikSolution (DMSO) can be added: 0 - 0.75uL per 25uL of reaction.
- ds-DNA template = 50ng per 25uL for <5 kb, or 100 ng per 25uL if for >5 kb.
- 2ng/uL works fine for 6kb plasmids. -Janet Matsen 9/2014
- primers:
- our lab usually dilutes primers to 10uM, and the recipes are for ng. Usually 10uM is ~100ng/uL.
- Add ~0.5uL of primer per 8uL of reaction. *Janet Matsen 9/2014
Thermocycling
- Regular Kit (not multi kit)
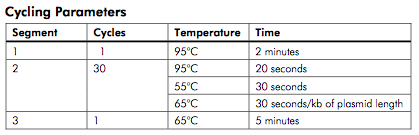
thermocycling protocol from Lightning manual
Tips
- Though the lightning Dpn1 enzyme claims to be effective in 5 minutes, you might as well incubate it for 30 minutes, just in case it lowers your unmutated background further.
- If you want to use one priming site to introduce mutations, it is about the same price to order 1 oligo and do it with the multi kit, or order 1 pair of complementary oligos and do it with the non-multi kit. If ordering less primers is appealing, go for it. A small decrease in efficiency might be seen, but it shouldn't be a problem based on JM's use of one primer to introduce 24 different mutations. (The first colony screened had the desired mutation in 90+% of the sequenced plasmids and the efficiency was quite high.)
- From the manual:
- Thaw the dNTP mix once, prepare single-use aliquots, and store the aliquots at –20°C. Do not subject the dNTP mix to multiple freeze-thaw cycles
- Make your kit last longer by doing 8uL reactions, not 25uL. You only transform 1uL per reaction so this is always plenty.