Lidstrom:Autoinduction Media
Return: Protocols
About Autoinduction media
- Glucose and the inducing sugar are present in the media while the cells grow. In the beginning, the cells prefer glucose. As the glucose runs out, the cells start taking up the induction sugar (usually lactose), causing induction of genes related to that sugar's metabolism (e.g. lac promoter, pTrc promoter, T7 promoter).
- Can also be done with arabinose or galactose.
The advantages are:
- you don't have to monitor OD of the cultures
- you can go straight from -80oC to protein purification in 1 day instead of two
- great for library screening where cultures have different growth rates
Use for T7 and pTrc
Instead of inducing a T7 or pTrc promoter with IPTG, autoinduction media can be used. Glucose, glycerol, and lactose are added to a buffered yeast broth to make autoinduction media. As the E. coli cultures grow, they consume the glucose first. As the glucose runs out, they are forced to use the lactose which drives expression of the T7 promoter. Glycerol helps support growth without inhibiting T7 protein expression.
Recipes from Sturdier 2014
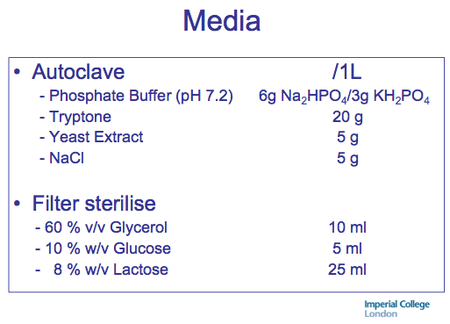
Recipe: Simplifed version from Imperial College (works great!)
Autoinduction Media recipe from Studier 2005. 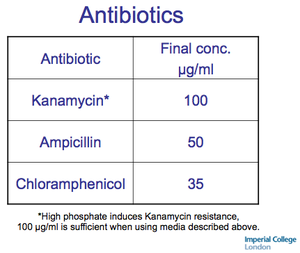
Autoinduction Media antibiotic specificiations. - Recipe in action; simplest possible version -JM
- No trace elements, added Mg. (usually not necessary)
- All sugars are dissolved and filtered together to maximize ease of use.
From Studier 2014
These are the official recipes, which take more time/energy to make. Start with the simplified recipe above and try this if the above method fails.
| Media | Inducing? | Purpose | Recipe | Final Concentration |
| ZYM-5052 | inducing | complex auto-inducing medium | 9.57 ml ZY, 20 μl 1 M MgSO4 (2 μl 1,000× metals, optional), 200 μl 50× 5052, 200 μl 50× M. | 1 % N-Z-amine AS, 0.5 % yeast extract, 25 mM Na 2 HPO 4 , 25 mM KH 2 PO4 , 50 mM NH 4 Cl, 5 mM Na2SO4 , 2 mM MgSO4 (0.2× metals, optional†), 0.5 % glycerol, 0.05 % glucose, 0.2 % α-lactose. |
† "Trace metals are generally not needed in complex media, but 0.2× metals could be added to ensure that metal requirements are met." - Studier 2014
Note: the notation is the hundredth of percent glycerol, the hundredth percent for glucose, and the tenth of percent inducing sugar (lactose, galactose)
- 5052 refers to 0.5% glycerol, 0.05% glucose, and 0.2% lactose
- 50 = 0.5% glycerol, 5 = 0.05% glucose, 2 = 0.2%lactose
| Component Stock | Recipe | 1X concentration | Notes | |
| ZY | 1 L water, 10 g tryptone (or "N-Z-amine AS"), 5 g yeast extract | tryptone = N-Z-amine AS, a soluble enzymatic digest of casein (milk). Use a brand such as Difco, Sigma, Fisher, etc. | ||
| 1 M MgSO4 | 87 ml water, 24.65 g MgSO4–7H2O | |||
| 1,000× metals | 1,000× metals | 50 mM FeCl 3, 20 mM CaCl 2 , 10 mM MnCl 2 , 10 mM ZnSO 4 , 2 mM CoCl 2 , 2 mM CuCl 2 , 2 mM NiCl 2 , 2 mM Na 2 MoO 4 , 2 mM Na 2 SeO 3 , 2 mM H 3 BO 3 , do not autoclave | The trace metal mix was assembled from autoclaved stock solutions of the individual components except for FeCl3, which was added from the 0.1 M solution in ~0.12 M HCl. ... | |
| 50× 5052 | 25 g glycerol (weigh in beaker), 73 ml water, 2.5 g glucose, 10 g α-lactose monohydrate | 0.5 % glycerol, 0.05 % glucose, 0.2 % α-lactose. | Helen's didn't dissolve 150410; added 50% more water, then it could be filtered. | |
| 50× M | 80 ml water, 17.75 g Na2HPO4 , 17.0 g KH2PO4 , 13.4 g NH4Cl, 3.55 g Na2SO4 . | 25 mM Na 2 HPO4 , 25 mM KH2PO4 , 50 mM NH4Cl, 5 mM Na2SO4 , pH ~6.7 ( see Note 1 ). | Occasionally 50× M has showered crystals upon standing at room temperature. They can be redissolved in the microwave |
References
- Original Paper: Studier FW (2005), Protein Production by Auto-Induction in High-Density Shaking Cultures. Protein Expr. Purif. 41(1): 207–234. <-- we make media ZYM-5052.
- A more practical recap of the original paper by the same author 7 years later: Stable Expression Clones and Auto-Induction for Protein Production in E. coli (free version (?))
- Practical summary slides: Imperial college .ppt slides: http://www3.imperial.ac.uk/pls/portallive/docs/1/15699698.PPT
Experiments
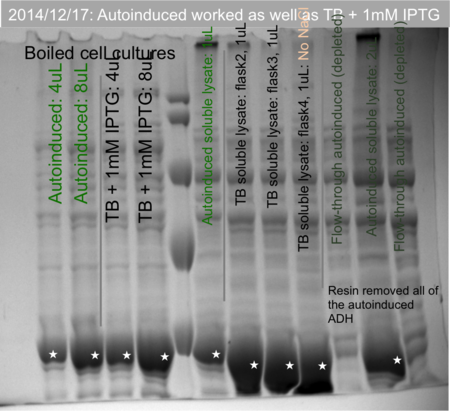
Comparing autoinduction media to TB induced with IPTG
Janet did an experiment in 12/2014 comparing cell pellets of His-tagged ADH grown in autoinduction media to the same culture grown in TB and induced with IPTG as part of this experiment. The autoinduced culture had comparable amounts of protein as seen by SDS-PAGE, and yielded more protein upon Co-NTA purification.
How does oxygenation affect protein production?
- Janet tested autoinduction media at different oxygenation levels to see how OD and protein/cell are affected. The protein grown was a His-tagged version of ACS 2P2F, which is relatively toxic. Good oxygenation was predicted to enhance expression because the toxicity of the protein is thought to be from depletion of the ATP pool when acetate is activated to acetyl-CoA.
- Full experiment details: document, spreadsheet.
- Cultures were grown in 50mL falcon tubes with the cap on, shaken on a floor shaker at 225 RPM at room temperature.
- Higher oxygenation (smaller culture volumes) resulted in ~3x higher OD *and* >3x higher protein/cell. That means there was ~10x more protein per mL of culture.

High oxygen --> ~3X higher OD - Note the OD is measured by NanoDrop; multiply the OD value by 10 to convert to 1cm cuvette measurements.
- This gel also shows a set of cultures where extra glycerol was added; this didn't impact OD or protein/cell.

High oxygen --> >3x higher protein/cell - These are OD normalized loadings of total cell protein for comparison of protein/cell.
Questions
Why is glycerol added?
"Glycerol is provided in auto-inducing media as a good carbon and energy source that does not prevent glucose depletion during growth, glucose exclusion of inducing sugars, or the uptake of inducing sugars upon glucose depletion." -- Sturdier 2014
How good does the oxygenation need to be?
See Stable Expression Clones and Auto-Induction for Protein Production in E. coli, which says you will get more protein with more oxygen. I think you still get good yields if they are fermenting but you will get even more if they aren't.
How does temperature affect the process?
See Stable Expression Clones and Auto-Induction for Protein Production in E. coli, which points out that oxygen is more soluble at lower temperatures. It may take longer to get to induction but the final OD and amount of protein produced may also be higher.
How to use other sugars (than lactose)
"The standard 0.2 % lactose selected initially for auto-inducing media [ 6 ] corresponds to 5.6 mM, which equates to 0.1 % galactose. Induction of the pBAD pro- moter in these media is effective at 0.05 % arabinose." -- Sturdier 2014
Other notes
- "Expression strains suitable for auto-induction must have functional transporters for the appropriate sugar. Induction by lactose requires active β-galactosidase to convert lactose to allolactose, the actual inducer, and a functional LacY transporter. Induction by galactose would not require active β-galactosidase, but the host strain must lack galactokinase activity and transport galactose well enough to achieve an intracellular concentration sufficient for inducing promoters blocked by lac repressor. IPTG is not suitable for use in auto-induction because it can enter the cell and induce expression without a specific transporter, and cultures cannot grow uninduced in the presence of IPTG." -- Sturdier 2014
- "Growth media contain 50 mM phosphate, which provides signifi cant buffering and supports growth to high densities."
- "The effectiveness of kanamycin as a selective agent against the growth of BL21(DE3) decreases with increasing concentration of phosphate in rich media [ 6 ]: 100 μg/ml of kanamycin is needed to assure killing in the media given here."
- Sturdier 2014 uses very high air:culture ratios:
- "Reasonably good aeration is important for maintaining meta- bolically balanced pH near neutral and obtaining growth to high cell densities. We typically grow cultures in a temperature- controlled rotary shaker at ~350 rpm, using vessels and volumes of culture that give approximately equivalent levels of aeration. For auto- induction of many cultures in parallel to test expres- sion and solubility, we grow 0.5 ml cultures in 13 × 100 mm glass culture tubes with plastic caps in the rotary shaker-incubator upright in plastic racks that hold up to 72 tubes. At the scale we work, this is much more convenient and controllable than using multi-well microtiter plates. However, microtiter plates can be used in high-throughput automation if aeration is properly addressed."
- "Moderate-scale auto-induction can use 400–500 ml of culture in 1.8-l baffled Fernbach flasks (Bellco)."
- "In general, auto-inducing cultures are inoculated with one-thousandth volume from a culture grown to saturation in non- inducing MDAG-135 from a freezer stock." -- Sturdier 2014
- The high dilution allows the entire culture to be growing uniformly by the time autoinduction begins.
- Cultures grown at 37 °C are typically grown overnight for 12–16 h, well past saturation. Saturation in ZYM- 5052 at 37 °C typically reaches an OD 600nm around 7–10 but can reach 20–30 in some highly expressing strains.
- Incubation for several hours at saturation after auto-induction usually has little effect on accumulation or solubility of target proteins.
- "To shorten the total incubation time, we typically grow cultures for a few hours at 37 °C until they become lightly turbid (less than OD 600nm ~1) and then transfer them to the lower temperature. It is a good idea to continue incubation of cultures grown overnight at low temperature and to read the culture density a few hours apart to be sure that they are saturated."
- "Auto-induction works over the entire temperature range from 18 to 37 °C, an advantage because some target proteins are substantially more soluble when expressed at lower temperatures. However, care must be taken to ensure that auto-induction is complete before harvesting cultures grown at the lower temperatures. Saturation densities are usually significantly higher than at 37 °C, presumably because of the higher solubility of oxygen at the lower temperatures."
- "In general, increasing the rate of aeration increases the density at which auto-induction begins and the density at which the culture saturates. Higher aeration also increases the minimum concentration of lactose needed for good induction. The standard 0.2 % lactose was chosen to be well in excess of that needed for good auto-induction over the range of conditions tested. Auto- induced culture densities greater than OD 600nm ~50 have been attained by using higher levels of glycerol, higher levels of aeration, and appropriate metabolic balancing of pH with aspartate or succinate."
- Antibiotics:
- "The antibiotic ampicillin is degraded by a secreted enzyme, β-lactamase, and is usually destroyed by the time turbidity becomes apparent in a culture, at which point cells that have lost plasmid can begin to overgrow the culture. Furthermore, enough β-lactamase can be produced and secreted that even a 200-fold dilution to grow a subculture can bring along enough enzyme to destroy the ampicillin present in the fresh medium and allow continued overgrowth of the culture. Kanamycin also had unanticipated problems. As pointed out in the first paragraph of Subheading 2.2 , kanamycin loses the ability to restrict the growth of BL21(DE3) (and presumably other E . coli strains as well) in rich media with commonly used phosphate concentrations. Once recognized, such problems can be avoided."
- If you have a super toxic protein, consider BL21 AI instead of BL21(DE3)
- "However, occasional target proteins are highly toxic to the cell
at extremely low concentrations. In the limit case where a single transcript of the target gene can generate enough protein to kill the cell, an expression plasmid could not be maintained in a culture unless the stochastic bursts of target protein from all the plasmids in the cell occurred at a frequency significantly lower than an aver- age of once per cell division. Basal expression of target protein will be reduced if basal expression of T7 RNA polymerase is reduced. This seems to be the case for BL21-AI, where T7 RNA polymerase appears to be produced from the uninduced pBAD promoter at a lower rate than in BL21(DE3) from the uninduced lacUV5 promoter. Indeed, some toxic target genes we have worked with were easier to establish and maintain in BL21-AI than in BL21(DE3), although"
- "The composition of a culture at any point in growth can also be determined by titering on four different plates [ 1 , 4 ]: (1) all viable cells will form colonies on an appropriate nutrient plate; (2) only cells that retain plasmid will form colonies on a plate containing the selective antibiotic; (3) cells that have lost plasmid or that cannot induce the target protein will give colonies on a plate with strong inducing capacity, such as an inducing concentration of IPTG where repression is maintained by lac repressor; and (4) mutant cells that retain plasmid but can- not induce target protein will give colonies on plates containing both the selective antibiotic and the inducing capacity. Using such a plating assay can help to determine where problems lie."
Medias described in Sturdier 2014
- MDAG-11 non-inducing agar plates for isolating transformants
More recipes from Studier 2014
Minimal
| Media | Inducing? | Purpose | Notes | Recipe | |
| MDAG-11 (w/ agarose) | no | non-inducing agar plates for isolating transformants | The lower concentration of glucose in MDAG-11 is to prevent standing cultures or colonies on agar plates from becoming too acidic from metabolism of excess glucose at low dissolved oxygen concentration. | 5 g agar, 475 ml H2O, autoclave for 15 min, mix well, let cool for ~10 min on bench or equilibrate in a 50 °C water bath. Add 1 ml 1 M MgSO 4 , 100 μl 1,000× metals, 1.25 ml 40 % glucose, 2 ml 25 % aspartate, 10 ml 50× M, 14 ml 18aa, and any nutrients required by the host cell (e.g., 50 μl 10 mM thiamine for XL1Blue-MR) or selective antibiotics (e.g., 2 ml of kanamycin stock solution, 25 mg/ml). Mix well, and pour ~20 ml per standard plastic Petri plate (pouring slowly until liquid just covers the bottom usually gives about the right amount per plate). This recipe makes ~25 plates with final composition of 1 % agar, 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO 4 , 0.2× metals, 0.1 % glucose, 0.1 % aspartate, 200 μg/ml of each of 18 amino acids (no C,Y) and optionally 1 μM thiamine, and 100 μg/ml kanamycin. | |
| MDAG-11 (w/o agarose) | no | non-inducing liquid | The lower concentration of glucose in MDAG-11 is to prevent standing cultures or colonies on agar plates from becoming too acidic from metabolism of excess glucose at low dissolved oxygen concentration. | 9.43 ml water, 20 μl 1 M MgSO 4 , 2 μl 1,000× metals, 25 μl 40 % glucose, 40 μl 25 % aspartate, 200 μl 50× M, 280 μl 18aa. Final composition: 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO 4 , 0.2× metals, 0.1 % glucose, 0.1 % aspartate, 200 μg/ml of each of 18 amino acids (no C,Y). | |
| MDAG-135 | no | non-inducing medium for growing high-density freezer stocks, working or seed cultures, or cultures for isolating plasmids | The combination of 0.35 % glucose, 0.1 % aspartate, and 200 μg/ml of each of 18 amino acids (0.36 %) in MDAG-135 was arrived at experimentally to provide metabolic balancing of pH at relatively high aeration so that cultures grow to high cell density and arrive at saturation near-neutral pH. Poor aeration should be avoided, as such cultures may become quite acidic. | 9.37 ml water, 20 μl 1 M MgSO4 , 2 μl 1,000× metals, 87.5 μl 40 % glucose, 40 μl 25 % aspartate, 200 μl 50× M, 280 μl 18aa. Final composition: 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO 4 , 0.2× metals, 0.35 % glucose, 0.1 % aspartate, 200 μg/ml of each of 18 amino acids (no C,Y). | |
| MDA-505 | variable | non-inducing medium for testing auto-induction by different concentrations of inducers | The fully defined non-inducing MDA-505 contains the same mixture of carbon and energy sources as the fully defined autoinducing MDA-5052 except for lack of an inducing sugar. An expression strain capable of producing large amounts of target protein grows to saturation in MDA-505 with no detectable induction, making this medium suitable for testing the effectiveness of different concentrations of inducing sugars. | 9.36 ml water, 20 μl 1 M MgSO 4 , 2 μl 1,000× metals, 100 μl 100× 505, 40 μl 25 % aspartate, 200 μl 50× M, 280 μl 18aa. Final composition: 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO 4 , 0.2× metals, 0.5 % glycerol, 0.05 % glucose, 0.1 % aspartate, 200 μg/ml of each of 18 amino acids (no C,Y). | |
| MDG | variable | non-inducing minimal medium | The only carbon and energy sources in MDG are 0.5 % glucose and 0.25 % aspartate for metabolic balancing of pH. Succinate can replace aspartate to make NH 4 the only source of nitrogen in this medium, and glycerol could replace glucose if desirable for labeling. BL21(DE3) grows well in this minimal medium | 9.55 ml water, 20 μl 1 M MgSO 4 , 2 μl 1,000× metals, 125 μl 40 % glucose, 100 μl 25 % aspartate, 200 μl 50× M. Final composi- tion: 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO 4 , 0.2× metals, 0.5 % glucose, 0.25 % aspartate. |
NOT Minimal
- I think the Y stands for yeast
| Media | Inducing? | Purpose | Recipe | Final Concentration |
| ZYM-505 | not inducing | General-Purpose Complex Medium for Rapid Growth to High Density | 9.68 ml ZY, 20 μl 1 M MgSO4 (2 μl 1,000× metals, optional), 100 μl 100× 505, 200 μl 50× M | 1 % N-Z-amine AS, 0.5 % yeast extract, 25 mM Na2HPO4, 25 mM KH2PO4, 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO4 (0.2× metals, optional), 0.5 % glycerol, 0.05 % glucose. |
Inducing media
All of these recipes except MD-5051 are formulated for autoinduction with 0.2 % lactose, which can be replaced for autoinduction with 0.1 % galactose, 0.05 % arabinose, or other sugars subject to glucose inhibition, as appropriate. Lactose and galactose can be exchanged by exchanging 50× 5052 (which provides 0.2 % lactose) and 50× 5051 (which provides 0.1 % galactose) in the recipes for auto-inducing media. Auto-induction of target genes under the control of the T7 lac promoter in BL21-AI requires both arabinose, to induce production of T7 RNA polymerase, and either lactose or galactose, to unblock the T7 lac promoter in the expression plasmid. 1.
| Media | Inducing? | Purpose | Recipe | Final Concentration |
| ZYM-5052 | inducing | complex auto-inducing medium | 9.57 ml ZY, 20 μl 1 M MgSO4 (2 μl 1,000× metals, optional), 200 μl 50× 5052, 200 μl 50× M. | 1 % N-Z-amine AS, 0.5 % yeast extract, 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na2SO4 , 2 mM MgSO4 (0.2× metals, optional), 0.5 % glycerol, 0.05 % glucose, 0.2 % α-lactose. |
| MDA-5052 | inducing | defined auto-inducing medium | 9.26 ml water, 20 μl 1 M MgSO4 , 2 μl 1,000× metals, 200 μl 50× 5052, 40 μl 25 % aspartate, 200 μl 50× M, 280 μl 18aa | 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO4 , 0.2× metals, 0.5 % glycerol, 0.05 % glucose, 0.2 % α-lactose, 0.1 % aspartate, 200 μg/ml of each of 18 amino acids (no C,Y) |
| MDA-5052 | inducing | defined auto-inducing medium | 9.26 ml water, 20 μl 1 M MgSO4 , 2 μl 1,000× metals, 200 μl 50× 5052, 40 μl 25 % aspartate, 200 μl 50× M, 280 μl 18aa | 25 mM Na 2 HPO 4 , 25 mM KH 2 PO 4 , 50 mM NH 4 Cl, 5 mM Na 2 SO 4 , 2 mM MgSO4 , 0.2× metals, 0.5 % glycerol, 0.05 % glucose, 0.2 % α-lactose, 0.1 % aspartate, 200 μg/ml of each of 18 amino acids (no C,Y) |
Note: the notation is the hundredth of percent glycerol, the hundredth percent for glucose, and the tenth of percent inducing sugar (lactose, galactose)
- The designation 505 refers to 0.5% glycerol, 0.05% glucose
- 50 = 0.5% glycerol, 5 = 0.05% glucose
- 5052 refers to 0.5% glycerol, 0.05% glucose, and 0.2% lactose
- 50 = 0.5% glycerol, 5 = 0.05% glucose, 2 = 0.2%lactose
- 5051 means 0.5 % glycerol, 0.05 % glucose, 0.1 % galactose.
- MDG: the D indicates 0.25% aspartate
| Component Stock | Recipe | 1X concentration | Notes |
| ZY | 1 L water, 10 g tryptone (or "N-Z-amine AS"), 5 g yeast extract | tryptone = N-Z-amine AS, a soluble enzymatic digest of casein in 100-lb barrels, and yeast extract (HY-YEST 444 in a 55-lb barrel) were obtained from Quest International, 5515 Sedge Blvd., Hoffman Estates, IL 60192, telephone 800-833-8308. These or equivalent materials (e.g., tryptone) are also available in various quantities from Difco, Sigma, Fisher, or other biochemical and chemical suppliers. | |
| 100× 505 | 50 g glycerol (weigh in beaker), 57 ml water, 5 g glucose | 0.5 % glycerol, 0.05 % glucose | |
| 50× M | 80 ml water, 17.75 g Na2HPO4 , 17.0 g KH2PO4 , 13.4 g NH4Cl, 3.55 g Na2SO4 . | 25 mM Na 2 HPO4 , 25 mM KH2PO4 , 50 mM NH4Cl, 5 mM Na2SO4 , pH ~6.7 ( see Note 1 ). | Occasionally 50× M has showered crystals upon standing at room temperature. They can be redissolved in the microwave |
Q&A from others
hydration of phosphates?
From Eric Mukherjee, 7/2015 about the simplest recipe:
It seems that if you assume they mean the heptahydrate for the Na2HPO4 and anhydrous KH2PO4, then you get 22mM of both KH2PO4. It looks like the protocol calls for 25mM of each of those. So it looks like it should be 6.8045g of KH2PO4 anhydrous in 2L and 13.4035 g Na2HPO4 heptahydrate in 2L total if you want 25mM (feel free to double check this).
importance of Mg2+?
From Dana Nadler, 5/2015:
When I tried the "simple" autoinduction recipe, I found that the final OD600 of the cultures was lower than I used to get with ZYM media. I retried the same strains with ZYM-5052 and got 2x-3x higher final OD600 (~7-9). My only guess to the difference is the lack of added Mg2+ in the simple formulation. This article is what made me think of this possible cause: http://schaechter.asmblog.org/schaechter/2009/11/the-limitations-of-lb-medium.html
So, you may want to try adding a bit of MgCl2 or MgSO4 to your simple recipe and see if it improves biomass/protein yield.