Lidstrom:Autoinduction Media: Difference between revisions
No edit summary |
|||
| Line 34: | Line 34: | ||
=== How does temperature affect the process? === | === How does temperature affect the process? === | ||
See [http://link.springer.com/protocol/10.1007%2F978-1-62703-691-7_2#page-1 Stable Expression Clones and Auto-Induction for Protein Production in E. coli], which points out that oxygen is more soluble at lower temperatures. It may take longer to get to induction but the final OD and amount of protein produced may also be higher. | See [http://link.springer.com/protocol/10.1007%2F978-1-62703-691-7_2#page-1 Stable Expression Clones and Auto-Induction for Protein Production in E. coli], which points out that oxygen is more soluble at lower temperatures. It may take longer to get to induction but the final OD and amount of protein produced may also be higher. | ||
== Other notes == | |||
* "Expression strains suitable for auto-induction must have functional transporters for the appropriate sugar. Induction by lactose requires active β-galactosidase to convert lactose to allolactose, the actual inducer, and a functional LacY transporter. Induction by galactose would not require active β-galactosidase, but the host strain must lack galactokinase activity and transport galactose well enough to achieve an intracellular concentration sufficient for inducing promoters blocked by lac repressor. IPTG is not suitable for use in auto-induction because it can enter the cell and induce expression without a specific transporter, and cultures cannot grow uninduced in the presence of IPTG." -- [http://www.ncbi.nlm.nih.gov/pubmed/24203322 Sturdier 2014] | |||
Revision as of 07:22, 31 March 2015
Return: Protocols
About Autoinduction media
- Glucose and the inducing sugar are present in the media while the cells grow. In the beginning, the cells prefer glucose. As the glucose runs out, the cells start taking up the induction sugar (usually lactose), causing induction of genes related to that sugar's metabolism (e.g. lac promoter, pTrc promoter, T7 promoter).
- Can also be done with arabinose or galactose.
Recipe:
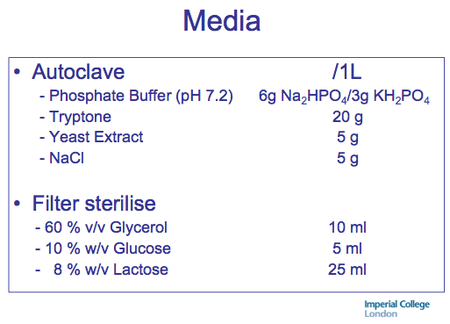
Autoinduction Media recipe from Studier 2005. 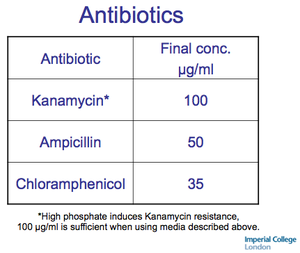
Autoinduction Media antibiotic specificiations. - Recipe in action -JM
Why Autoinduction Media?
Instead of inducing a T7 promoter with IPTG, autoinduction media can be used. Glucose, glycerol, and fructose are added to a buffered yeast broth to make autoinduction media. As the E. coli cultures grow, they consume the glucose first. As the glucose runs out, they are forced to use the fructose which drives expression of the T7 promoter. Glycerol helps support growth without inhibiting T7 protein expression.
The advantages are:
- you don't have to monitor OD of the cultures
- you can go straight from -80oC to protein purification in 1 day instead of two
- great for library screening where cultures have different growth rates
References
- Original Paper: Studier FW (2005), Protein Production by Auto-Induction in High-Density Shaking Cultures. Protein Expr. Purif. 41(1): 207–234. <-- we make media ZYM-5052.
- A more practical recap of the original paper by the same author 7 years later: Stable Expression Clones and Auto-Induction for Protein Production in E. coli (free version (?))
- Practical summary slides: Imperial college .ppt slides: http://www3.imperial.ac.uk/pls/portallive/docs/1/15699698.PPT
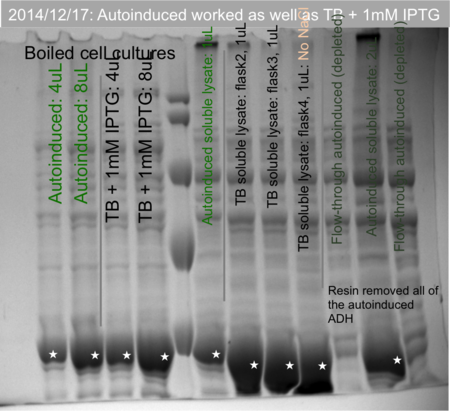
Comparing autoinduction media to TB induced with IPTG
Janet did an experiment in 12/2014 comparing cell pellets of His-tagged ADH grown in autoinduction media to the same culture grown in TB and induced with IPTG as part of this experiment. The autoinduced culture had comparable amounts of protein as seen by SDS-PAGE, and yielded more protein upon Co-NTA purification.
Questions
How good does the oxygenation need to be?
See Stable Expression Clones and Auto-Induction for Protein Production in E. coli, which says you will get more protein with more oxygen. I think you still get good yields if they are fermenting but you will get even more if they aren't.
How does temperature affect the process?
See Stable Expression Clones and Auto-Induction for Protein Production in E. coli, which points out that oxygen is more soluble at lower temperatures. It may take longer to get to induction but the final OD and amount of protein produced may also be higher.
Other notes
- "Expression strains suitable for auto-induction must have functional transporters for the appropriate sugar. Induction by lactose requires active β-galactosidase to convert lactose to allolactose, the actual inducer, and a functional LacY transporter. Induction by galactose would not require active β-galactosidase, but the host strain must lack galactokinase activity and transport galactose well enough to achieve an intracellular concentration sufficient for inducing promoters blocked by lac repressor. IPTG is not suitable for use in auto-induction because it can enter the cell and induce expression without a specific transporter, and cultures cannot grow uninduced in the presence of IPTG." -- Sturdier 2014