Janet B. Matsen:Lab Tips & Tricks: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
(→Gels) |
|||
| (19 intermediate revisions by the same user not shown) | |||
| Line 2: | Line 2: | ||
I came to the Lidstrom Lab with zero molecular biology or synthetic biology experience. I have great appreciation for all of the resources put online by the OpenWetWare community and other websites, so I am hopeful that I can share some of what I have learned. Please contact [[User:Janet B. Matsen | me]] with questions, comments, and corrections. | I came to the Lidstrom Lab with zero molecular biology or synthetic biology experience. I have great appreciation for all of the resources put online by the OpenWetWare community and other websites, so I am hopeful that I can share some of what I have learned. Please contact [[User:Janet B. Matsen | me]] with questions, comments, and corrections. | ||
== DNA Magic == | == DNA Magic == | ||
===Minipreps=== | ===Minipreps=== | ||
====Grow in TB, not LB==== | |||
*This alone gives me orders of magnitude higher miniprep yields. | |||
====Grow overnight cultures with sufficient head space==== | |||
*the air volume should be at least 4 times the liquid volume. | |||
* increasing the culture volume seems like a good idea at first but the lower oxygen concentrations are detrimental to plasmid yields. | |||
*Specific suggestions for amounts of culture grow pSB plasmids overnight before miniprepping: | *Specific suggestions for amounts of culture grow pSB plasmids overnight before miniprepping: | ||
**pSB1A3 (high copy number): 2mL of TB is sufficient for miniprepping. 5 mL in a 15 mL test tube is great. | **pSB1A3 (high copy number): 2mL of TB is sufficient for miniprepping. 5 mL in a 15 mL test tube is great. | ||
**pSB3K3 (medium copy number): 5+ mL in green-capped test tubes (they are 25+ mL) | **pSB3K3 (medium copy number): 5+ mL in green-capped test tubes (they are 25+ mL) | ||
**pSB4C5: I have only done pSB4C5 (low copy number) once I prepped from 50 mL TB (overnight in a 250 mL flask) and got a very pleasing 200 ng/uL in 50 uL. The absorbance at 230nm looked higher than usual but the DNA peak was good. Now I usually do 5-10 mL in a green-capped tube, but have never gotten 200 ng/uL! | **pSB4C5: I have only done pSB4C5 (low copy number) once I prepped from 50 mL TB (overnight in a 250 mL flask) and got a very pleasing 200 ng/uL in 50 uL. The absorbance at 230nm looked higher than usual but the DNA peak was good. Now I usually do 5-10 mL in a green-capped tube, but have never gotten 200 ng/uL! | ||
==== The strain you use really matters! ==== | |||
* I do most of my minipreps from Top10 strains. If you use other strains, make sure they are cloning strains. Cloning strains have nucleases knocked out, making your miniprep more stable. | |||
* In 10/2012 I tried miniprepping DNA from BL21(ai) and doing a restriction digest. The DNA was totally degraded afterward. I compared the phenotypes of Top10 and BL21(ai) using [http://openwetware.org/wiki/E._coli_genotypes#BL21.28AI.29 this list] and found that Top10 has EndA1 knocked out, whereas BL21(ai) didn't. | |||
* Elizabeth "Betsy" Skovran taught Amanda to use [http://www.qiagen.com/products/accessories/buffers/bufferpb.aspx PB buffer]. The composition is proprietary. We use this on our Thermo Scientific miniprep kit: add 500 uL after loading DNA. | |||
===PCR=== | ===PCR=== | ||
*Optimizing PCR rxns: | *Optimizing PCR rxns: | ||
**Try the PCR with 55oC for annealing and 0% DMSO first, almost always. If that doesn't work, you will see the manual gives you MANY options for things to change including template concentration, primer concentration, annealing temperature, Mg2+ concentrations, polymerase concentration, alternate buffers, extension time, and annealing temperature. There is no way you could test all the combinatorial possibilities here. I generally only change the annealing temperature and % DMSO if my template concentration is within the suggested range. | **Try the PCR with 55oC for annealing and 0% DMSO first, almost always. If that doesn't work, you will see the manual gives you MANY options for things to change including template concentration, primer concentration, annealing temperature, Mg2+ concentrations, polymerase concentration, alternate buffers, extension time, and annealing temperature. There is no way you could test all the combinatorial possibilities here. I generally only change the annealing temperature and % DMSO if my template concentration is within the suggested range. | ||
*** When Tm = 55oC failed, I used to do 12 rxns (20 uL each) with 4 temperatures (on a gradient PCR block), each at 4 temperatures ranging from 55oC - 70oC, and 0%, 5%, and 10% DMSO. If one of these conditions don't work, there is little hope that changing other things will be a game changer. Now that feels like too much work & I only do a few temps w/o DMSO. | *** When Tm = 55oC failed, I used to do 12 rxns (20 uL each) with 4 temperatures (on a gradient PCR block), each at 4 temperatures ranging from 55oC - 70oC, and 0%, 5%, and 10% DMSO. If one of these conditions don't work, there is little hope that changing other things will be a game changer. Now that feels like too much work & I only do a few temps w/o DMSO. | ||
* If you are unsure whether you added an ingredient to the PCR mix, add it again. | |||
** I *always* have a written list of components and the volume I want to add and I pen in a dot next to ingredients I have added. However, it is possible that you can get distracted and be unsure whether you added a component. If that component is template, nucleotides, or enzyme, I always add it again. I have never been unsure about whether I added buffer or DMSO (when desired) so I can't promise performance if you double these concentrations. | |||
* I also add the most abundant & inexpensive components to the mix first. Thus, I add water, buffer, then primers. If I am unsure that something is wrong at this point, I can scrap it without any lost of scarce or valuable materials. | |||
* If making more than one master mix, label the tubes of master mix by writing the number of the primers on the lid. Then you can easily double check that the right primer goes in the right mix as you create it. | |||
** I don't actually do this because I like only having 1 kit. Keeps things simple. | |||
*Annealing @ 55oC in PCR before anything else often works great. You are more likely to get undesired bands due to mis-priming but it is often a great place to start. | |||
===Gels=== | ===Gels=== | ||
*You can re-use tips when loading into wells if you rinse in the TB buffer in your block between every step. This is great because tips are ~$40/box. Don't do this for important samples (just in case.) | *You can re-use tips when loading into wells if you rinse in the TB buffer in your block between every step. This is great because tips are ~$40/box. Don't do this for important samples (just in case.) | ||
*If you are gel- | *If you are gel-purifying large volumes of PCR product, you can tape the lanes together. The greatest dangers are (a) the well ripping and allowing your sample to leak out and (b) having the buffer slosh you sample out of the well when you move or bump the box. Come talk to me about extremes in taping because I have made all the possible mistakes. | ||
** I don't actually do this any more (2012/07/02) because I am using Gibson cloning and don't need much DNA any more. | ** I don't actually do this any more (2012/07/02) because I am using Gibson cloning and don't need much DNA any more. | ||
*Running large volumes on a gel ("overloading" the gel) | *Running large volumes on a gel ("overloading" the gel) can cause the band size to run too fast or too slow. If you are running a large amount for purification, include a lane next to the ladder that is not overloaded so you can be confident you know the band size. The following gel ran alright, but a slight shift is visible. | ||
[[image:purification gel.jpg|thumb|center|include small not-overloaded lanes when running purification gels]] | [[image:janet purification gel.jpg|thumb|center|include small not-overloaded lanes when running purification gels]] | ||
===Purifying DNA === | |||
*purifying DNA on a gel column has ~ 90% loss. purifying DNA on a column (non-gel) has ~ 90% recovery. avoid gel purification. (Justin) | |||
*Freeze 'N Squeeze Gel extraction may be worth a try. | |||
** I'd like to try. I even have enough of a kit to try some, but I never have samples I feel like taking the risk on! The only person I know who does it (also the person who gave me a piece of a kit) swears by it. | |||
*Purify gels on gel-specific kits. Purify PCR products on PCR product specific kits if you have one. The former does a less effective job removing primer dimers. (Justin) | |||
==== Eluting DNA from miniprep & gel extraction columns ==== | |||
* By default, most people elute from miniprep and gel purification columns in water instead of the supplied buffer. This is motivated by the desire to have DNA that is free of buffers and other compounds that may inhibit subsequent reactions (e.g. PCR or Gibson Assembly). You may, however, suffer from lower recovery of DNA from your column as the pH of the water you use is likely < 7.5. | |||
* Both the Qiagen [http://www.qiagen.com/faq/faqview.aspx?faqid=199&SearchText=&FaqCategoryId=0&MenuItemId=0&catalog=1&ProductLineId=1000252 gel extraction kit] and Thermo Scientific [http://www.fermentas.com/templates/files/tiny_mce/coa_pdf/coa_k0502.pdf miniprep kit] use 10 mM Tris·Cl , pH 8.5 elution buffer. | |||
** Should not inhibit PCR. (No EDTA, a chelator) | |||
** Gibson assembly mix is ~ 200 mM Tris, pH 7.5. Adding a few uL of 10 mM pH 8.5 Tris shouldn't have a huge effect. I'd like to call their tech support and make sure, however. | |||
** Is ligation the main issue? I'm not doing a lot of restriction cloning right now, so this wouldn't be a huge issue for me. (10/2012) | |||
== Equipment == | == Equipment == | ||
| Line 40: | Line 59: | ||
==Media== | ==Media== | ||
*TB (terrific broth) gives you healthier cells and more plasmids than LB. I use LB for freezer stocks because or media prep person makes it in abundance but TB for minipreps to get higher plasmid yield. | *TB (terrific broth) gives you healthier cells and more plasmids than LB. I use LB for freezer stocks because or media prep person makes it in abundance but TB for minipreps to get higher plasmid yield. | ||
*SOB media ("super optimal broth") can be used like TB. The recipes are somewhat variable. | |||
*SOC media is SOB with added glucose; it can be used when cells recover from transformation. | |||
** Our lab and Rob Egbert haven't needed to use it as of 2012/10/3. But I am considering making some SOC for electroporation. | |||
==Transformations== | ==Transformations== | ||
| Line 56: | Line 78: | ||
** I name them by their plasmid number & short description. | ** I name them by their plasmid number & short description. | ||
** In this, I keep an extra copy of the sequence file that is annotate with all of the confirming sequencing rxns I did. For each sequence that comes back, I add a feature that is named with the order number of the sequencing reaction & the primer that was used to obtain the sequence. The feature should cover only the part of the sequence for which the sequencing reaction was solid. This means visually trimming off areas with N's. | ** In this, I keep an extra copy of the sequence file that is annotate with all of the confirming sequencing rxns I did. For each sequence that comes back, I add a feature that is named with the order number of the sequencing reaction & the primer that was used to obtain the sequence. The feature should cover only the part of the sequence for which the sequencing reaction was solid. This means visually trimming off areas with N's. | ||
== Using APE (free plasmid editing software) == | |||
*Pros: | |||
** Free --> can have it on all of your computers | |||
** Allows annotation libraries --> Easy annotation of new plasmids | |||
*About the annotation libraries: | |||
** This is one of the most useful tools. You can edit them in any text editor. To use them, use the drop-down menus to load the library, then click to annotate your current sequence using this library. | |||
** I keep an annotation library that holds all of my sequencing primers, and use this to sequence clones. I open my plasmid annotated with genes, etc., then load this library of primers and overlay the primers on top of my sequence. If you have annotations of the regions of your plasmid that you have sequence verification for, it makes it very easy to find primers that will sequence other regions you are interested in. | |||
*Beware: | |||
** When you are searching for a sequence in your APE file, it is possible that it will incorrectly tell you it is not there | |||
*** This is happening because the DNA is stored a linear, not circular. If you have the cursor clicked mid-way through the file, the "find next" button will only look in the portion of the sequence that is between your cursor and the last letter. This can be avoided by storing all DNA as circular. There is a rectangular button in the upper right corner you can click until it says "circular." | |||
** The alignment tool has given me ... unusual... results. Instead, I mostly use [http://blast.ncbi.nlm.nih.gov/Blast.cgi?PROGRAM=blastn&BLAST_PROGRAMS=megaBlast&PAGE_TYPE=BlastSearch&SHOW_DEFAULTS=on&LINK_LOC=blasthome BLAST] and use the blastn option. | |||
*** Note: FASTA format needs to have a space after the > | |||
== Misc == | == Misc == | ||
*Don't be surprised if you get contamination on plates with multiple antibiotic resistances. There are plenty of plasmids floating around natural systems with multiple on them already. Bacteria make antibiotics in natural systems to add competition to an an environment. (Mary Lidstrom, 2012) | *Don't be surprised if you get contamination on plates with multiple antibiotic resistances. There are plenty of plasmids floating around natural systems with multiple on them already. Bacteria make antibiotics in natural systems to add competition to an an environment. (Mary Lidstrom, 2012) | ||
Revision as of 10:49, 29 October 2012
Back to Janet
I came to the Lidstrom Lab with zero molecular biology or synthetic biology experience. I have great appreciation for all of the resources put online by the OpenWetWare community and other websites, so I am hopeful that I can share some of what I have learned. Please contact me with questions, comments, and corrections.
DNA Magic
Minipreps
Grow in TB, not LB
- This alone gives me orders of magnitude higher miniprep yields.
Grow overnight cultures with sufficient head space
- the air volume should be at least 4 times the liquid volume.
- increasing the culture volume seems like a good idea at first but the lower oxygen concentrations are detrimental to plasmid yields.
- Specific suggestions for amounts of culture grow pSB plasmids overnight before miniprepping:
- pSB1A3 (high copy number): 2mL of TB is sufficient for miniprepping. 5 mL in a 15 mL test tube is great.
- pSB3K3 (medium copy number): 5+ mL in green-capped test tubes (they are 25+ mL)
- pSB4C5: I have only done pSB4C5 (low copy number) once I prepped from 50 mL TB (overnight in a 250 mL flask) and got a very pleasing 200 ng/uL in 50 uL. The absorbance at 230nm looked higher than usual but the DNA peak was good. Now I usually do 5-10 mL in a green-capped tube, but have never gotten 200 ng/uL!
The strain you use really matters!
- I do most of my minipreps from Top10 strains. If you use other strains, make sure they are cloning strains. Cloning strains have nucleases knocked out, making your miniprep more stable.
- In 10/2012 I tried miniprepping DNA from BL21(ai) and doing a restriction digest. The DNA was totally degraded afterward. I compared the phenotypes of Top10 and BL21(ai) using this list and found that Top10 has EndA1 knocked out, whereas BL21(ai) didn't.
- Elizabeth "Betsy" Skovran taught Amanda to use PB buffer. The composition is proprietary. We use this on our Thermo Scientific miniprep kit: add 500 uL after loading DNA.
PCR
- Optimizing PCR rxns:
- Try the PCR with 55oC for annealing and 0% DMSO first, almost always. If that doesn't work, you will see the manual gives you MANY options for things to change including template concentration, primer concentration, annealing temperature, Mg2+ concentrations, polymerase concentration, alternate buffers, extension time, and annealing temperature. There is no way you could test all the combinatorial possibilities here. I generally only change the annealing temperature and % DMSO if my template concentration is within the suggested range.
- When Tm = 55oC failed, I used to do 12 rxns (20 uL each) with 4 temperatures (on a gradient PCR block), each at 4 temperatures ranging from 55oC - 70oC, and 0%, 5%, and 10% DMSO. If one of these conditions don't work, there is little hope that changing other things will be a game changer. Now that feels like too much work & I only do a few temps w/o DMSO.
- Try the PCR with 55oC for annealing and 0% DMSO first, almost always. If that doesn't work, you will see the manual gives you MANY options for things to change including template concentration, primer concentration, annealing temperature, Mg2+ concentrations, polymerase concentration, alternate buffers, extension time, and annealing temperature. There is no way you could test all the combinatorial possibilities here. I generally only change the annealing temperature and % DMSO if my template concentration is within the suggested range.
- If you are unsure whether you added an ingredient to the PCR mix, add it again.
- I *always* have a written list of components and the volume I want to add and I pen in a dot next to ingredients I have added. However, it is possible that you can get distracted and be unsure whether you added a component. If that component is template, nucleotides, or enzyme, I always add it again. I have never been unsure about whether I added buffer or DMSO (when desired) so I can't promise performance if you double these concentrations.
- I also add the most abundant & inexpensive components to the mix first. Thus, I add water, buffer, then primers. If I am unsure that something is wrong at this point, I can scrap it without any lost of scarce or valuable materials.
- If making more than one master mix, label the tubes of master mix by writing the number of the primers on the lid. Then you can easily double check that the right primer goes in the right mix as you create it.
- I don't actually do this because I like only having 1 kit. Keeps things simple.
- Annealing @ 55oC in PCR before anything else often works great. You are more likely to get undesired bands due to mis-priming but it is often a great place to start.
Gels
- You can re-use tips when loading into wells if you rinse in the TB buffer in your block between every step. This is great because tips are ~$40/box. Don't do this for important samples (just in case.)
- If you are gel-purifying large volumes of PCR product, you can tape the lanes together. The greatest dangers are (a) the well ripping and allowing your sample to leak out and (b) having the buffer slosh you sample out of the well when you move or bump the box. Come talk to me about extremes in taping because I have made all the possible mistakes.
- I don't actually do this any more (2012/07/02) because I am using Gibson cloning and don't need much DNA any more.
- Running large volumes on a gel ("overloading" the gel) can cause the band size to run too fast or too slow. If you are running a large amount for purification, include a lane next to the ladder that is not overloaded so you can be confident you know the band size. The following gel ran alright, but a slight shift is visible.

Purifying DNA
- purifying DNA on a gel column has ~ 90% loss. purifying DNA on a column (non-gel) has ~ 90% recovery. avoid gel purification. (Justin)
- Freeze 'N Squeeze Gel extraction may be worth a try.
- I'd like to try. I even have enough of a kit to try some, but I never have samples I feel like taking the risk on! The only person I know who does it (also the person who gave me a piece of a kit) swears by it.
- Purify gels on gel-specific kits. Purify PCR products on PCR product specific kits if you have one. The former does a less effective job removing primer dimers. (Justin)
Eluting DNA from miniprep & gel extraction columns
- By default, most people elute from miniprep and gel purification columns in water instead of the supplied buffer. This is motivated by the desire to have DNA that is free of buffers and other compounds that may inhibit subsequent reactions (e.g. PCR or Gibson Assembly). You may, however, suffer from lower recovery of DNA from your column as the pH of the water you use is likely < 7.5.
- Both the Qiagen gel extraction kit and Thermo Scientific miniprep kit use 10 mM Tris·Cl , pH 8.5 elution buffer.
- Should not inhibit PCR. (No EDTA, a chelator)
- Gibson assembly mix is ~ 200 mM Tris, pH 7.5. Adding a few uL of 10 mM pH 8.5 Tris shouldn't have a huge effect. I'd like to call their tech support and make sure, however.
- Is ligation the main issue? I'm not doing a lot of restriction cloning right now, so this wouldn't be a huge issue for me. (10/2012)
Equipment
- Check out my rebound board for discarding used tips! I won't be shooting tips onto Amanda's bench any more.

Media
- TB (terrific broth) gives you healthier cells and more plasmids than LB. I use LB for freezer stocks because or media prep person makes it in abundance but TB for minipreps to get higher plasmid yield.
- SOB media ("super optimal broth") can be used like TB. The recipes are somewhat variable.
- SOC media is SOB with added glucose; it can be used when cells recover from transformation.
- Our lab and Rob Egbert haven't needed to use it as of 2012/10/3. But I am considering making some SOC for electroporation.
Transformations
- Plate transformations by spreading with a bent 20 uL pipette tip, not an ethanol/flame sterilized stick, glass beads, etc. You have to use the pipette tip anyway to slurp up some of the cells, so just bend it using the inside of the eppendorf tube and use it to spread the cells.
- I have light a small fire using a flame-sterilized stick, and roller beads are a hassle and hard to get non-uniform spreading of cells with. I like nonuniform spreading because it will allow you to grab single colonies even if your transformation efficiency is really high or really low.
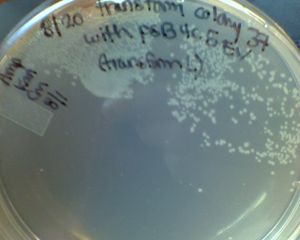
- Don't transform plates at room temp over the weekend, especially if you rely on Amp resistance. Often you see a lot of contamination! (Janet)
Keeping primers, plasmids, strain stocks, & sequencing organized
- Keep a master google spreadsheet with a page each for primers, plasmids, strain stocks, and sequencing. (example) This will keep you very organized, and allow you to collaborate more easily.
- On the primers page, each primer should have an individual number, a name without spaces, the sequence, comments for its intended use, and the date you ordered it. When ordering primers from IDT, you can paste the three consecutive columns with primer number, name, & sequence into the multiple primer entry window. Then delete the space between the primer number & name, and add a hyphen. You can grey out primers you decide are no longer useful (example: you find out it is wrong or doesn't work).
- On the plasmids page...
- On the strain stocks page...
- Keep the primers, plasmid stocks, cell stocks, and strain stocks in boxes with their unique identification number on top. Order them by number. This allows for ease of sharing resources & information. Note that the plasmids can be stored as minipreps and in the cloning strains, so you will have two boxes that share the same numbers.
- Keep a dropbox folder with the final versions of all of the plasmids you trust & keep.
- I name them by their plasmid number & short description.
- In this, I keep an extra copy of the sequence file that is annotate with all of the confirming sequencing rxns I did. For each sequence that comes back, I add a feature that is named with the order number of the sequencing reaction & the primer that was used to obtain the sequence. The feature should cover only the part of the sequence for which the sequencing reaction was solid. This means visually trimming off areas with N's.
Using APE (free plasmid editing software)
- Pros:
- Free --> can have it on all of your computers
- Allows annotation libraries --> Easy annotation of new plasmids
- About the annotation libraries:
- This is one of the most useful tools. You can edit them in any text editor. To use them, use the drop-down menus to load the library, then click to annotate your current sequence using this library.
- I keep an annotation library that holds all of my sequencing primers, and use this to sequence clones. I open my plasmid annotated with genes, etc., then load this library of primers and overlay the primers on top of my sequence. If you have annotations of the regions of your plasmid that you have sequence verification for, it makes it very easy to find primers that will sequence other regions you are interested in.
- Beware:
- When you are searching for a sequence in your APE file, it is possible that it will incorrectly tell you it is not there
- This is happening because the DNA is stored a linear, not circular. If you have the cursor clicked mid-way through the file, the "find next" button will only look in the portion of the sequence that is between your cursor and the last letter. This can be avoided by storing all DNA as circular. There is a rectangular button in the upper right corner you can click until it says "circular."
- The alignment tool has given me ... unusual... results. Instead, I mostly use BLAST and use the blastn option.
- Note: FASTA format needs to have a space after the >
- When you are searching for a sequence in your APE file, it is possible that it will incorrectly tell you it is not there
Misc
- Don't be surprised if you get contamination on plates with multiple antibiotic resistances. There are plenty of plasmids floating around natural systems with multiple on them already. Bacteria make antibiotics in natural systems to add competition to an an environment. (Mary Lidstrom, 2012)