IGEM:Imperial/2010/Detection module/schistosoma: Difference between revisions
No edit summary |
|||
| Line 8: | Line 8: | ||
Infected people can pass out several thousands of parasite eggs in their stool (most species) or urine (''Schistosoma haematobium''). These eggs were first discovered in Egypt by Theodor Maximilian Bilharz, a German pathologist. Under the microscope one can see that they are around 110-170 µm long by 40 to 70 µm wide giving them an elongated shape with a distinctive terminal spine that is used for identification. | Infected people can pass out several thousands of parasite eggs in their stool (most species) or urine (''Schistosoma haematobium''). These eggs were first discovered in Egypt by Theodor Maximilian Bilharz, a German pathologist. Under the microscope one can see that they are around 110-170 µm long by 40 to 70 µm wide giving them an elongated shape with a distinctive terminal spine that is used for identification. | ||
[[Image:Schistosoma egg.png|thumb|left|200px| Egg of S. ''haematobium''. [http://www.stanford.edu/class/humbio103/ParaSites2004/Schisto/website.html Stanford University (2004)]]] | |||
These characteristically shaped eggs are viable for around week and once in contact with water they quickly hatch and release the first larval form called miracidia. These free-swimming, ciliated larvae cannot feed and will usually die within 24 hours if they fail to detect a suitable intermediary host, using light and chemical clues, and subsequently enter it. Different schistosoma species show preference for different host species but at this stage all rely on fresh water snails. | These characteristically shaped eggs are viable for around week and once in contact with water they quickly hatch and release the first larval form called miracidia. These free-swimming, ciliated larvae cannot feed and will usually die within 24 hours if they fail to detect a suitable intermediary host, using light and chemical clues, and subsequently enter it. Different schistosoma species show preference for different host species but at this stage all rely on fresh water snails. | ||
| Line 14: | Line 16: | ||
This process takes between 4 and 6 weeks from which time on thousands of cercaria, a second larval form, can be shed from the snails daily for several month. this shedding is induced by light [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4KY3RPD-M&_user=217827&_coverDate=09%2F29%2F2006&_alid=1505007608&_rdoc=5&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=4886&_sort=r&_st=13&_docanchor=&view=c&_ct=9&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=27498d6154512f9cccda7ff0d2fbb805&searchtype=a (Gryseels ''et al.'' 2006)] and aims to maximise the likelihood of cercaria finding their second host. The larvae can survive in the water for up to 72 hours, although more commonly around 30 hours, during which time they use chemical stimuli as well as light and temperature to detect the skin of a suitable host. By far the most powerful stimulus to triggering invasive behaviour are skin lipids, in particular medium-chain free fatty acids [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6W7G-45NG5GM-2&_user=217827&_coverDate=05%2F01%2F2002&_alid=1505006308&_rdoc=1&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6626&_sort=r&_st=13&_docanchor=&view=c&_ct=2&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=a339daf27956149b4080f0c6f1d2169c&searchtype=a (McKerrow and Salter 2002)]: Of the C18 fatty acids examined, stearic (18:0) is inactive, oleic (18:1) slightly active, linoleic (18:2) and linolenic (18:3) acids highly active [http://journals.cambridge.org/action/displayAbstract;jsessionid=B1A531CD8298B78F36E5A5A336522AD3.tomcat1?fromPage=online&aid=4180708 (Austin ''et al.'' 1974)]. Once detected by the parasite these stimuli lead to signal-dependent breakdown of inositol phospholipids which is directly linked to activation of protein kinase C (via elevated diacylglycerol level) and mobilization of calicium (via elevated levels of inositol triphosphate) which in turn evokes subsequent cellular response [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GG2-FP&_user=217827&_coverDate=04%2F30%2F1991&_alid=1505009318&_rdoc=1&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=4&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=b5b1e3b6d044a907895dc267d542cf09&searchtype=a (Matsumura ''et al.'' 1991)] such as the release of enzymes from a specialized gland - called acetabular gland complex – at the posterior region of the head of the parasite [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GPD-KH&_user=217827&_coverDate=08%2F31%2F1992&_alid=1505013773&_rdoc=2&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=8&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=273e73fe67245cd2feb84a18cc1fbbec&searchtype=a (Fishelson ''et al.'' 1992)]. | This process takes between 4 and 6 weeks from which time on thousands of cercaria, a second larval form, can be shed from the snails daily for several month. this shedding is induced by light [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4KY3RPD-M&_user=217827&_coverDate=09%2F29%2F2006&_alid=1505007608&_rdoc=5&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=4886&_sort=r&_st=13&_docanchor=&view=c&_ct=9&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=27498d6154512f9cccda7ff0d2fbb805&searchtype=a (Gryseels ''et al.'' 2006)] and aims to maximise the likelihood of cercaria finding their second host. The larvae can survive in the water for up to 72 hours, although more commonly around 30 hours, during which time they use chemical stimuli as well as light and temperature to detect the skin of a suitable host. By far the most powerful stimulus to triggering invasive behaviour are skin lipids, in particular medium-chain free fatty acids [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6W7G-45NG5GM-2&_user=217827&_coverDate=05%2F01%2F2002&_alid=1505006308&_rdoc=1&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6626&_sort=r&_st=13&_docanchor=&view=c&_ct=2&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=a339daf27956149b4080f0c6f1d2169c&searchtype=a (McKerrow and Salter 2002)]: Of the C18 fatty acids examined, stearic (18:0) is inactive, oleic (18:1) slightly active, linoleic (18:2) and linolenic (18:3) acids highly active [http://journals.cambridge.org/action/displayAbstract;jsessionid=B1A531CD8298B78F36E5A5A336522AD3.tomcat1?fromPage=online&aid=4180708 (Austin ''et al.'' 1974)]. Once detected by the parasite these stimuli lead to signal-dependent breakdown of inositol phospholipids which is directly linked to activation of protein kinase C (via elevated diacylglycerol level) and mobilization of calicium (via elevated levels of inositol triphosphate) which in turn evokes subsequent cellular response [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GG2-FP&_user=217827&_coverDate=04%2F30%2F1991&_alid=1505009318&_rdoc=1&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=4&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=b5b1e3b6d044a907895dc267d542cf09&searchtype=a (Matsumura ''et al.'' 1991)] such as the release of enzymes from a specialized gland - called acetabular gland complex – at the posterior region of the head of the parasite [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6WFH-4C52GPD-KH&_user=217827&_coverDate=08%2F31%2F1992&_alid=1505013773&_rdoc=2&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=6795&_sort=r&_st=13&_docanchor=&view=c&_ct=8&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=273e73fe67245cd2feb84a18cc1fbbec&searchtype=a (Fishelson ''et al.'' 1992)]. | ||
[[Image:Cercaria.jpg|thumb|left|200px| Cercaria [http://biology.unm.edu/biology/esloker/pi/SchistoEvolHistory.htm Loker Laboratory 2006]]] | [[Image:Cercaria.jpg|thumb|left|200px| Cercaria [http://biology.unm.edu/biology/esloker/pi/SchistoEvolHistory.htm Loker Laboratory (2006)]]] | ||
These enzymes include number of proteases that target proteins in the host's skin such as keratin and elastin the latter of which is cleaved by the most essential and abundant protease: schistosomal elastase that we use in our iGEM project to detect schistosoma. This process allows cercaria to burrow into the skin of their new host, where they leave their tail behind. | These enzymes include number of proteases that target proteins in the host's skin such as keratin and elastin the latter of which is cleaved by the most essential and abundant protease: schistosomal elastase that we use in our iGEM project to detect schistosoma. This process allows cercaria to burrow into the skin of their new host, where they leave their tail behind. | ||
There are six species of schistosoma known to successfully infect humans: ''Schistosoma mansoni'' , which uses ''Biomphalaria'' snails as intermediate host and can cause intestinal and hepatic schistosomiasis in Africa, the Arabian peninsula as well as South America; ''S. japonicum'', a zoonotic parasite, which uses ''Oncomelania'' snails as intermediate host and can cause intestinal and hepatosplenic schistosomiasis, especially in South-East Asia; ''S. mekongi'', ''S. intercalatum'' and related ''S. guineansis'' ,which are restricted to small areas an only of local importance; as well as ''S. haematobium'', transmitted by ''Bulinus'' snails, which can give rise to urinary schistosomiasis in Africa and the Arabian peninsula | There are six species of schistosoma known to successfully infect humans: ''Schistosoma mansoni'' , which uses ''Biomphalaria'' snails as intermediate host and can cause intestinal and hepatic schistosomiasis in Africa, the Arabian peninsula as well as South America; ''S. japonicum'', a zoonotic parasite, which uses ''Oncomelania'' snails as intermediate host and can cause intestinal and hepatosplenic schistosomiasis, especially in South-East Asia; ''S. mekongi'', ''S. intercalatum'' and related ''S. guineansis'' ,which are restricted to small areas an only of local importance; as well as ''S. haematobium'', transmitted by ''Bulinus'' snails, which can give rise to urinary schistosomiasis in Africa and the Arabian peninsula | ||
[[Image:Spread of schistosoma species (Gryseels et al. 2006).png|thumb|center|750px| Map of the distribution and spread of schistosoma species. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4KY3RPD-M&_user=217827&_coverDate=09%2F29%2F2006&_alid=1505007608&_rdoc=5&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=4886&_sort=r&_st=13&_docanchor=&view=c&_ct=9&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=27498d6154512f9cccda7ff0d2fbb805&searchtype=a (Gryseels et al. 2006)]]] | [[Image:Spread of schistosoma species (Gryseels et al. 2006).png|thumb|center|750px| Map of the distribution and spread of schistosoma species. [http://www.sciencedirect.com/science?_ob=ArticleURL&_udi=B6T1B-4KY3RPD-M&_user=217827&_coverDate=09%2F29%2F2006&_alid=1505007608&_rdoc=5&_fmt=high&_orig=search&_origin=search&_zone=rslt_list_item&_cdi=4886&_sort=r&_st=13&_docanchor=&view=c&_ct=9&_acct=C000011279&_version=1&_urlVersion=0&_userid=217827&md5=27498d6154512f9cccda7ff0d2fbb805&searchtype=a (Gryseels et al. 2006)]]] | ||
Revision as of 06:35, 21 October 2010
Schistosomiasis

Schistosoma, from the class of trematodes constitute a genus commonly known as blood-flukes, are the parasites causing a disease called bilharzias or schistosomiasis which, according to the WHO (2010) is amongst the most devastating parasitic disease, second only to malaria, with 207 million infected and around 700 million at risk. To understand the disease itself, as well as the possible approaches to improving its control we have to gain a deeper understanding of the complex life cycle of this parsite. Like many other eukryotic parasites, the schistosoma life cycle involving several stages in and outside two different hosts.
The Life Cycle
Infected people can pass out several thousands of parasite eggs in their stool (most species) or urine (Schistosoma haematobium). These eggs were first discovered in Egypt by Theodor Maximilian Bilharz, a German pathologist. Under the microscope one can see that they are around 110-170 µm long by 40 to 70 µm wide giving them an elongated shape with a distinctive terminal spine that is used for identification.

These characteristically shaped eggs are viable for around week and once in contact with water they quickly hatch and release the first larval form called miracidia. These free-swimming, ciliated larvae cannot feed and will usually die within 24 hours if they fail to detect a suitable intermediary host, using light and chemical clues, and subsequently enter it. Different schistosoma species show preference for different host species but at this stage all rely on fresh water snails. Once inside the host the miracidia reproduce asexually and form multicellular sporocysts which later develop into cercarial larvae with embryonic suckers and a characteristic bifurcated tail.
This process takes between 4 and 6 weeks from which time on thousands of cercaria, a second larval form, can be shed from the snails daily for several month. this shedding is induced by light (Gryseels et al. 2006) and aims to maximise the likelihood of cercaria finding their second host. The larvae can survive in the water for up to 72 hours, although more commonly around 30 hours, during which time they use chemical stimuli as well as light and temperature to detect the skin of a suitable host. By far the most powerful stimulus to triggering invasive behaviour are skin lipids, in particular medium-chain free fatty acids (McKerrow and Salter 2002): Of the C18 fatty acids examined, stearic (18:0) is inactive, oleic (18:1) slightly active, linoleic (18:2) and linolenic (18:3) acids highly active (Austin et al. 1974). Once detected by the parasite these stimuli lead to signal-dependent breakdown of inositol phospholipids which is directly linked to activation of protein kinase C (via elevated diacylglycerol level) and mobilization of calicium (via elevated levels of inositol triphosphate) which in turn evokes subsequent cellular response (Matsumura et al. 1991) such as the release of enzymes from a specialized gland - called acetabular gland complex – at the posterior region of the head of the parasite (Fishelson et al. 1992).

These enzymes include number of proteases that target proteins in the host's skin such as keratin and elastin the latter of which is cleaved by the most essential and abundant protease: schistosomal elastase that we use in our iGEM project to detect schistosoma. This process allows cercaria to burrow into the skin of their new host, where they leave their tail behind.
There are six species of schistosoma known to successfully infect humans: Schistosoma mansoni , which uses Biomphalaria snails as intermediate host and can cause intestinal and hepatic schistosomiasis in Africa, the Arabian peninsula as well as South America; S. japonicum, a zoonotic parasite, which uses Oncomelania snails as intermediate host and can cause intestinal and hepatosplenic schistosomiasis, especially in South-East Asia; S. mekongi, S. intercalatum and related S. guineansis ,which are restricted to small areas an only of local importance; as well as S. haematobium, transmitted by Bulinus snails, which can give rise to urinary schistosomiasis in Africa and the Arabian peninsula

Additionally there is number of avian schistosoma species, especially in Northern Europe, the USA and Canada, that cannot infect humans but will still try to enter our skin giving rise to the harmless but unpleasant condition called swimmer's itch.
Once inside the human body, the cercaria travel through the blood via the lungs into the portal vein of the liver where they develop into schistosomulae. After a maturation period one to one and a half month after which they mate. The way schistosoma have evolved to mate is unique amongst the trematodes in that there is a significant sexual dimorphism, which also inspired their name which translates from Greek to 'split body'. This name describes the male anatomy as there is a long groove, called gynecophoral canal, along its body in which the female is held.
Adult males are between 10 to 15 mm long whereas females are longer, between 16 to 22mm, and thinner. Both sexes have two suckers – one on each end – to hold onto the walls of blood vessels where they feed on blood and globulins through anaerobic glycolysis. The debris is regurgitated in the host’s blood. After mating they migrate to their perivesicular (S. Haematobium) or mesenteric (other species) destination where the female releases her eggs. With an average life span of 3 to 5 years, although up to 30 years is possible,” the theoretical reproduction potential of one schistosome pair is up to 600 billion schistosomes” (Gryseels et al. 2006).
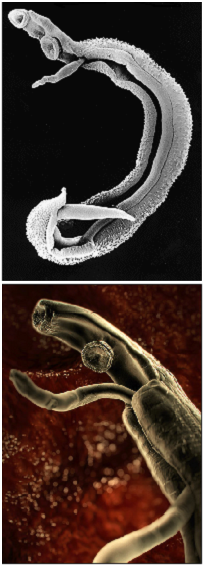
The eggs themselves secrete proteolytic enzymes that allow the eggs to reach their destination: the bladder (S. Haematobium) or intestines (other species) from which they are released by the host into the environment, allowing the cycle to continue.
Impact on human health
Symptoms
Long-term effects
Epidemiology
Treatment
Praziquantel
There are a number of drugs available to cure infection with schistosomes, for example amoscanate, chloroxylenol, meclonazepam and oxamniquine. However the by far most widely used helminticide to combat schistosomiasis is Praziquantel despite not being licensed for medical treatment of humans in the UK. It is effective agains schistosoma in a single treatment and is also used for some other parasitic trematodes such as liver flukes. Although the drug has been in use for over 20 years, the mechanism of its action is still not understood (Aragon et al. 2009).
"Praziquantel is the recommended treatment for schistosomiasis at 40 mg/kg body weight. The cost of a single 600-mg tablets is about US$ 0.08 and an average treatment is estimated to be between US$ 0.20–0.30. Praziquantel is now available free of charge to a few high-disease burden least developed countries (LDC), through a donation from Merck KGaA to the World Health Organization. The donation of praziquantel is based on a successful review of the national plan for schistosomiasis control and a commitment of resources for implementation." (WHO, 2010)
Communal approach to treatment
Because of the difficult nature of schistosomiasis diagnosis, treatment is often not provided after individual diagnosis, but instead it is very common to test only a sample of individuals in a community, especially school children, and if a certain proportion of the sampled people is infected, the whole community is treated with praziquantel. This measure allows control of the disease to some extend if done in a coordinated approach with treatment on a large scale with safe and effective drug, and at regular intervals (WHO, 2010). The communal approach aims at reducing the late stages of schistosomiasis that go along with malnutrition and often cause developmental problem in children. Based on the sampled disease burden is also the length of the period after which treatment is repeated. This method will not eradicate the disease but rather to lower the total number of infected people and prevent severe infection in order to reduce morbidity as well as socio-economic impact. In theory it is also possible to break the parasitic life cycle with this method and permanently prevent reinfection, however in practise it is well known that eradication does not take place for a number of different reasons, which is why treatment is repeated regularly. Even if schistosoma were completely eradicated from one area, due to the continuing presence of the water snail vector, recolonialisation by schistosoma would take place rapidly.
The Schistosomiasis Control Initiative based at Imperial College is involved in treatment of this and six other NTD in sub-Saharan Africa. Professor Alan Fenwick and (NAME) have also been a great help in the development of our project so if you are interested in their work, please follow the following link: Schistosomiasis Control Initiative - Homepage