Haynes Lab:Notebook/Characterizing AHL quorum sensing homologs/2013/10/28: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
Rene M Davis (talk | contribs) |
Rene M Davis (talk | contribs) |
||
| Line 6: | Line 6: | ||
| colspan="2"| | | colspan="2"| | ||
<!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | <!-- ##### DO NOT edit above this line unless you know what you are doing. ##### --> | ||
== | ==10/28/2013== | ||
Miniprepped J04450 that I started growing on Saturday. They were in the incubator for a long time and were super red. <br> | Miniprepped J04450 that I started growing on Saturday. They were in the incubator for a long time and were super red. <br> | ||
Revision as of 16:17, 2 December 2013
| <html><img src="/images/9/94/Report.png" border="0" /></html> Main project page <html><img src="/images/c/c3/Resultset_previous.png" border="0" /></html>Previous entry<html> </html>Next entry<html><img src="/images/5/5c/Resultset_next.png" border="0" /></html> | |||||||||
10/28/2013Miniprepped J04450 that I started growing on Saturday. They were in the incubator for a long time and were super red.
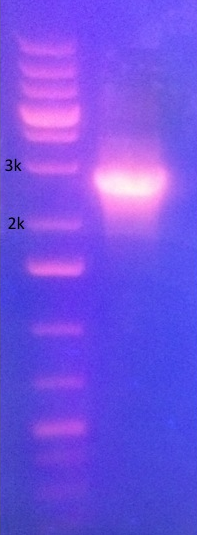
Restriction digest Gel of cut J04450 PCR need to edit this to include the next lane. lane 1 is P087/P088 and lane 2 is P089/P090, both I13522
2
3
| |||||||||