Haynes:NewProtocol: Difference between revisions
From OpenWetWare
Jump to navigationJump to search
Rene M Davis (talk | contribs) No edit summary |
Rene M Davis (talk | contribs) No edit summary |
||
| Line 8: | Line 8: | ||
==Overview== | ==Overview== | ||
<div style="width: 800px"> | <div style="width: 800px"> | ||
The Gibson Assembly method allows you to assemble multiple DNA parts in just 2 steps. | The Gibson Assembly method allows you to assemble multiple DNA parts in just 2 steps.<br> | ||
In the first step, 20 basepair overlaps are added to the sequences to be assembled: | |||
[[image:Slide1.png|500px|Gibson Assembly step 1]] <br> | |||
In the second step, the PCR products are added to the Gibson Assembly Mix: | |||
[[image:Slide2.jpg|500px|Gibson Assembly step 2]] | |||
==Materials== | ==Materials== | ||
Designing primers: | |||
[[Image:Slide_3_pdf.png|500px|Gibson Assembly primers]]<br> | |||
For a 50 μL PCR reaction: | For a 50 μL PCR reaction: | ||
| Line 52: | Line 62: | ||
</biblio>--> | </biblio>--> | ||
<!-- Try the [[Template:FormatRef|FormatRef template]]--> | <!-- Try the [[Template:FormatRef|FormatRef template]]--> | ||
#{{FormatRef| | #{{FormatRef|Gibson, DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO |2009| |Nature Methods 6(5)|343-5| }} PMID 19363495 | ||
==Contact== | ==Contact== | ||
Revision as of 11:24, 26 October 2012
Gibson Assembly
Overview
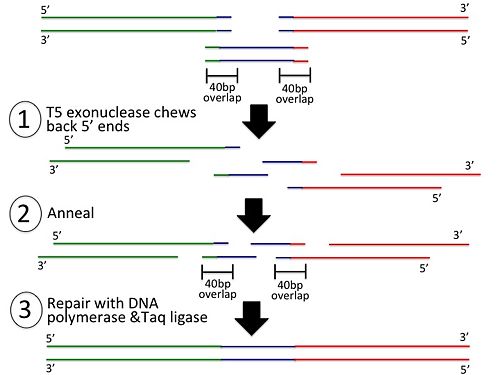
The Gibson Assembly method allows you to assemble multiple DNA parts in just 2 steps.
In the first step, 20 basepair overlaps are added to the sequences to be assembled:
In the second step, the PCR products are added to the Gibson Assembly Mix:
Materials
Designing primers:
For a 50 μL PCR reaction:
- 35 μL H2O
- 5 μL 10X PCR buffer
- 5 μL 2mM dNTPs (each)
- 1.5 μL 50mM MgCl2
- 1 μL 50μM sense primer
- 1 μL 50μM antisense primer
- 1 μL 5nM DNA template
- 0.5 μL TAQ DNA polyermerase
Procedure
- In a PCR tube, mix the components on ice in the order they are listed above.
- Perform thermocycling program
- 95 °C 5 min
- 95 °C 30 s
- TH 30 s
- 72 °C 1 min for each 1 kb PCR product
- Repeat steps 2-4 a total of 12-36 times (24 is standard).
- 72 °C 5 min
- 12 °C hold
Notes
Please feel free to post comments, questions, or improvements to this protocol. Happy to have your input!
- List troubleshooting tips here.
- You can also link to FAQs/tips provided by other sources such as the manufacturer or other websites.
- Anecdotal observations that might be of use to others can also be posted here.
Please sign your name to your note by adding '''*~~~~''': to the beginning of your tip.
References
Relevant papers and books
- Gibson, DG, Young L, Chuang RY, Venter JC, Hutchison CA, Smith HO (2009) - Nature Methods 6(5) 343-5 PMID 19363495
Contact
- René at rene.davis at asu dot edu
or instead, discuss this protocol.