Frankel:Force Spectroscopy: Difference between revisions
No edit summary |
No edit summary |
||
| Line 34: | Line 34: | ||
---- | ---- | ||
'''<font color=#000000 font size=3> Force spectra taken on raised terraces and lower features. | '''<font color=#000000 font size=3> | ||
Rupture force distribution of self assembled gp160 unfolding on terraced and lower regions. | Force spectra taken on raised terraces and lower features. Rupture force distribution of self assembled gp160 unfolding on terraced and lower regions. | ||
Rupture forces were measured as 79.6 ± 3.9 pN and 81.3 ± 3.8 pN for the terraces and lower regions, respectively. These forces are much lower than those measured for unfolding of isolated proteins on mica, which were above 160 pN. The lower unfolding forces suggest that GP160 is considerably easier to unfold when aggregated than isolated.</font>''' | Rupture forces were measured as 79.6 ± 3.9 pN and 81.3 ± 3.8 pN for the terraces and lower regions, respectively. These forces are much lower than those measured for unfolding of isolated proteins on mica, which were above 160 pN. The lower unfolding forces suggest that GP160 is considerably easier to unfold when aggregated than isolated.</font>''' | ||
| Line 53: | Line 53: | ||
|} | |} | ||
|- | |- | ||
| align=" | | align="center" style="border: 2px solid #000000; background-color:#FFFFFF; padding:1em;" valign="top"| | ||
'''<font color=#000000 font size=3> Revealing the selective interactions of fibronectin with lipid bilayer. </font>''' | '''<font color=#000000 font size=3> Revealing the selective interactions of fibronectin with lipid bilayer. </font>''' | ||
[[Image:FNsawt.png|300px]] | [[Image:FNsawt.png|300px]] | ||
[[Image:HisFN.png|300px]] | [[Image:HisFN.png|300px]] | ||
| Line 67: | Line 66: | ||
---- | ---- | ||
'''<font | '''<font align="justify" font size=3> | ||
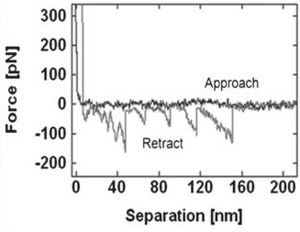
Sawtooth pattern on the retraction force curve indicating the unfolding of fibronectin. The average rupture force distribution of the protein on mica surface was 85.1 ± 2.7 pN. | |||
</font>''' | </font>''' | ||
Revision as of 20:24, 8 November 2012
<owwmenu align="center" font="helvetica" bold="1" color="white" bgcolor="black" hovercolor="black" bghovercolor="orange" topfontsize="10" fontSize="10" image="Danbanner-bio-machines.jpg" >
Home=Frankel Members=#,Principal Investigator=Frankel:Lab_Members, PhD students=Frankel:Lab_Members, Alumni=Frankel:Lab_Members Contact=Frankel:Contact Collaborators=Frankel:Collaborators Publications=Frankel:Publications Lab=Frankel:Research Research=#,Force Spectroscopy=Frankel:Force Spectroscopy,HIV/Virus=Frankel:HIV/Virus,ECM Proteins=Frankel:ECM Proteins,Cyberplasm=Frankel:Cyberplasm,Cancer=Frankel:Cancer
<startFeed/>
Force Spectroscopy
|
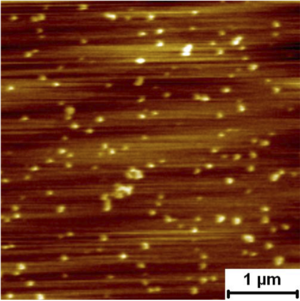
Assembly Self assembly and pore formation of HIV GP160 revealed at molecular resolution GP160mica Force spectra taken on raised terraces and lower features. Rupture force distribution of self assembled gp160 unfolding on terraced and lower regions. Rupture forces were measured as 79.6 ± 3.9 pN and 81.3 ± 3.8 pN for the terraces and lower regions, respectively. These forces are much lower than those measured for unfolding of isolated proteins on mica, which were above 160 pN. The lower unfolding forces suggest that GP160 is considerably easier to unfold when aggregated than isolated.
| ||
|
Revealing the selective interactions of fibronectin with lipid bilayer.
Sawtooth pattern on the retraction force curve indicating the unfolding of fibronectin. The average rupture force distribution of the protein on mica surface was 85.1 ± 2.7 pN. |