Frankel:ECM Proteins: Difference between revisions
No edit summary |
No edit summary |
||
| Line 1: | Line 1: | ||
{{Frankel}} | {{Frankel}} | ||
<br /> | |||
<br /> | |||
<div style="padding: align="center" 10px; width: 800px; border: 5px solid white;"> | <div style="padding: align="center" 10px; width: 800px; border: 5px solid white;"> | ||
Revision as of 13:24, 9 November 2012
<owwmenu align="center" font="helvetica" bold="1" color="white" bgcolor="black" hovercolor="black" bghovercolor="orange" topfontsize="10" fontSize="10" image="Danbanner-bio-machines.jpg" >
Home=Frankel Members=#,Principal Investigator=Frankel:Lab_Members, PhD students=Frankel:Lab_Members, Alumni=Frankel:Lab_Members Contact=Frankel:Contact Collaborators=Frankel:Collaborators Publications=Frankel:Publications Lab=Frankel:Research Research=#,Force Spectroscopy=Frankel:Force Spectroscopy,HIV/Virus=Frankel:HIV/Virus,ECM Proteins=Frankel:ECM Proteins,Cyberplasm=Frankel:Cyberplasm,Cancer=Frankel:Cancer
ECM Proteins
|
| ||
|
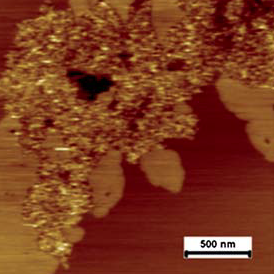
One of the major components of the extracellular matrix (ECM) is fibronectin, this dimeric glycoprotein is involved in numerous cell processes and has important functions in vertebrate development. Fibronectin in the extracellular matrix interacts with the transmembrane integrins in a highly specific manner. The ECM ligand protein contains a tripeptide recognition site, Arg-Gly-Asp (RGD), responsible for the affinity of the cell surface receptors with its ligand. Atomic force microscopy images can be appreciated, these illustrations depict how fibronectin molecules are almost exclusively absorbed on the DPPC domains. |