Collagen Biomaterials by Hieu La: Difference between revisions
No edit summary |
|||
| (6 intermediate revisions by one other user not shown) | |||
| Line 1: | Line 1: | ||
{{Template:ChemEng590B}} | |||
== Background == | == Background == | ||
Collagen is the main component of connective tissues and the most abundant protein in | Collagen is the main component of connective tissues and the most abundant protein in animals, accounting for 25% of the total protein mass [1]. It is the most abundant protein that can be found in the extracellular matrix and is mostly found in fibrous tissues. The term collagen refers to not a singular protein, but a wide family of proteins. To date, there are 28 types of collagen which have been identified. Collagen is commonly produced in fibroblasts [2]. | ||
Collagen biomaterials can be organized into two major groups depending on their processing. The first group is a decellurized collagen matrix which preserves the original tissue shape and ECM structure. The other group is based off of extracted and purified collagen which is then reformed in a functional scaffold [3]. | Collagen biomaterials can be organized into two major groups depending on their processing. The first group is a decellurized collagen matrix which preserves the original tissue shape and ECM structure. The other group is based off of extracted and purified collagen which is then reformed in a functional scaffold [3]. | ||
| Line 23: | Line 24: | ||
Collagen of various types forms the foundation of many tissues found in living organisms. Part of collagen’s appeal as a biomaterial is its natural abundance and that it can be extracted from the tissue of almost any animal. Collagen from bovine skin, porcine skin, and rat tail are most commonly used for biomedical applications [6]. | Collagen of various types forms the foundation of many tissues found in living organisms. Part of collagen’s appeal as a biomaterial is its natural abundance and that it can be extracted from the tissue of almost any animal. Collagen from bovine skin, porcine skin, and rat tail are most commonly used for biomedical applications [6]. | ||
Due to its compact structure, collagen fibers have remarkable mechanical properties and stability. In addition, collagen’s structure make the protein resistant to proteolysis and enzymatic degradation at neutral pH. However, its degradation in vivo can be manipulated via pH change making it an easily controllable property based on the application [1]. Collagen has also been used as a biomaterial due to low toxicity and immunogenic reactions. DeLustro et al. investigated the immunogenicity of various collagen products and found that the dominant immunological response was due to the small quantities of non-collagenous proteins present [7]. This can be attributed to the similarity in amino acid sequence in collagen among species. Animal collagen in the form of sutures or hemostatic agents is considered safe and making it suitable for use as a biomaterial. | Due to its compact structure, collagen fibers have remarkable mechanical properties and stability. In addition, collagen’s structure make the protein resistant to proteolysis and enzymatic degradation at neutral pH. However, its degradation in vivo can be manipulated via pH change making it an easily controllable property based on the application. Collagen based materials can often be absorbed into the human body after their purpose has been completed [1]. Collagen has also been used as a biomaterial due to low toxicity and immunogenic reactions. DeLustro et al. investigated the immunogenicity of various collagen products and found that the dominant immunological response was due to the small quantities of non-collagenous proteins present [7]. This can be attributed to the similarity in amino acid sequence in collagen among species. Animal collagen in the form of sutures or hemostatic agents is considered safe and making it suitable for use as a biomaterial. | ||
At first, collagen was believed to have purely a role in supporting structure in biological systems; however, researchers discovered that collagen has a role in many cellular functions including growth, differentiation, and migration [6]. It has been demonstrated that collagen can enhance the growth of different cell types, and type I collagen substrates have been used for the culture of various cells [8]. Hugues et al. showed that adhesion to collagen molecules is necessary in order for platelet activation and enhance wound healing [9]. | At first, collagen was believed to have purely a role in supporting structure in biological systems; however, researchers discovered that collagen has a role in many cellular functions including growth, differentiation, and migration [6]. It has been demonstrated that collagen can enhance the growth of different cell types, and type I collagen substrates have been used for the culture of various cells [8]. Hugues et al. showed that adhesion to collagen molecules is necessary in order for platelet activation and enhance wound healing [9]. | ||
| Line 60: | Line 61: | ||
==== Dentistry ==== | ==== Dentistry ==== | ||
Due to the fact that collagen can enhance wound healing via clot formation and stabilization, it has seen use in healing applications following dental procedures. Experiments conducted by Pitaru et al. indicate that type I collagen is capable of supporting regeneration of periodontal tissues [18]. A multitude of collagen based products are available for the control of bleeding post-dental surgery. Products are soft, white, sponge-like structures that when applied to a bleeding surface rapidly absorb blood and create an artificial clot. They achieve hemostasis within 2 to 5 minutes and if left in place will completely resorb within 56 days. Collagen films have been used as a replacement for sutures due to its chemical resemblance to connective tissue and the fact it does not hinder the healing process. Collagen-based scaffolds have been used in order to facilitate the growth of endothelial cells. [19] Collagen membranes have also been used as barriers to the migration of epithelial cells to encourage healing by providing room for regenerative cells in guided tissue regeneration (GTR) processes. A variety of collagen membranes of type I and type III are available commercially for GTR [16]. Collagen films and sponges are both used as temporary and permanent wound dressings in dental applications. The three dimensional structure of sponges composed of large pores allow for cell migration into them and tissue growth. Crosslinked collagen films on a methacrylate substrate are another form of collagen used as oral wound dressings [19]. | Due to the fact that collagen can enhance wound healing via clot formation and stabilization, it has seen use in healing applications following dental procedures. Experiments conducted by Pitaru et al. indicate that type I collagen is capable of supporting regeneration of periodontal tissues [18]. A multitude of collagen based products are available for the control of bleeding post-dental surgery. Products are soft, white, sponge-like structures that when applied to a bleeding surface rapidly absorb blood and create an artificial clot. They achieve hemostasis within 2 to 5 minutes and if left in place will completely resorb within 56 days. Collagen films have been used as a replacement for sutures due to its chemical resemblance to connective tissue and the fact it does not hinder the healing process. Collagen-based scaffolds have been used in order to facilitate the growth of endothelial cells. [19] Collagen membranes have also been used as barriers to the migration of epithelial cells to encourage healing by providing room for regenerative cells in guided tissue regeneration (GTR) processes. A variety of collagen membranes of type I and type III are available commercially for GTR [16]. Collagen films and sponges are both used as temporary and permanent wound dressings in dental applications. The three dimensional structure of sponges composed of large pores allow for cell migration into them and tissue growth. Crosslinked collagen films on a methacrylate substrate are another form of collagen used as oral wound dressings [19]. | ||
[[Image:Coldent.jpg|thumb||left|upright=1.0|alt= Various commercially available collagen-based dental implants|Various commercially available collagen-based dental implants [19]]] | |||
==== Tissue Engineering ==== | ==== Tissue Engineering ==== | ||
| Line 67: | Line 70: | ||
Collagen scaffolds are used to study cell migration, proliferation, and differentiation. They have also been used to study in vitro how cells behave in complex environments. Collagen scaffolds can be produced using a variety of methods in order to allow access to cell membranes, model the nervous system, or allow for the investigation between cancer cells and other cell types in a 3D environment. Scaffold can also be hardened via mineralization in order to allow for their use in bone and cartilage regeneration [3]. | Collagen scaffolds are used to study cell migration, proliferation, and differentiation. They have also been used to study in vitro how cells behave in complex environments. Collagen scaffolds can be produced using a variety of methods in order to allow access to cell membranes, model the nervous system, or allow for the investigation between cancer cells and other cell types in a 3D environment. Scaffold can also be hardened via mineralization in order to allow for their use in bone and cartilage regeneration [3]. | ||
== Challenges == | |||
In most collagen extraction and purification methods, intramolecular crosslinking is lost which results in decreased strength of the material. In order to obtain collagen of additional strength, additional processing steps must be undergone in order to restore cross-links [6]. | |||
The presence of collagen can also cause changes in cell growth or movement within biological tissues. As cells can interact with collagen easily, cells can pull and reorganize collagen fibers resulting in shrinkage or loss of shape in collagen scaffolds [3]. | |||
Collagen molecules can occasionally cause an immune response when they are foreign to a host organism. This immune response is due to the production of antibodies to the foreign collagen and may cause organ damage. Studies have shown that collagen types III, V, and VI lead to a much larger antibody response than type I collagen. There are three locations on the collagen molecule which can be targeted for antibody creation, on the terminal non-helical telopeptide ends of the molecule, and a central point known as the helical (A-) determinant. As such, these areas must be either removed or hidden in collagen processing before it is suitable for implantation or injection. For one commercially available collagen implant, Zyderm®, 3% of patients experienced elevated anti-implant antibody levels, while another 2% reacted in response to a second injection [17]. These patients experienced localized inflammation as a result of their implants. | |||
== Future Directions == | == Future Directions == | ||
[[Image:Col3.jpg|thumb|upright=1.0|alt=A composite collagen PLGA porous scaffold |A composite collagen PLGA porous scaffold [19]]] | |||
Biomaterial research as a whole has moved from focusing on singular materials to composite materials in order to create biomaterials with desirable properties of both. In particular, collagen and hydroxyapatite composite materials are being investigated due to the high mechanical properties of hydroxyapatite and the cellular interactions of collagen. Another research focus is on the physical properties of collagen in bones and whether they can be manipulated to increase fracture resistance [20]. | Biomaterial research as a whole has moved from focusing on singular materials to composite materials in order to create biomaterials with desirable properties of both. In particular, collagen and hydroxyapatite composite materials are being investigated due to the high mechanical properties of hydroxyapatite and the cellular interactions of collagen. Another research focus is on the physical properties of collagen in bones and whether they can be manipulated to increase fracture resistance [20]. | ||
Latest revision as of 20:35, 15 April 2016
Background
Collagen is the main component of connective tissues and the most abundant protein in animals, accounting for 25% of the total protein mass [1]. It is the most abundant protein that can be found in the extracellular matrix and is mostly found in fibrous tissues. The term collagen refers to not a singular protein, but a wide family of proteins. To date, there are 28 types of collagen which have been identified. Collagen is commonly produced in fibroblasts [2].
Collagen biomaterials can be organized into two major groups depending on their processing. The first group is a decellurized collagen matrix which preserves the original tissue shape and ECM structure. The other group is based off of extracted and purified collagen which is then reformed in a functional scaffold [3].
Structure
![The structure of collagen [3]](https://s3-us-west-2.amazonaws.com/oww-files-thumb/1/18/Collstrunew.PNG/300px-Collstrunew.PNG)
The similarity linking the 28 types of collagen is three polypeptide chains in a characteristic right handed triple helical structure. The strands are intertwined around each other similarly to rope. Various types of collagen differ in segments that interrupt the triple helical structure and fold in different three dimensional shapes. While the three polypeptide strands can be identical, different strands are more commonly found in collagen. Two common amino acid triplets are glycine-proline-X and glycine-X-hydroxyproline, where X is another amino acid. The high glycine content of collagen allows for tight packing in the tertiary and quaternary structure of collagen molecules due to the presence of hydrogen bonds. The fundamental unit of collagen molecules is a rod shaped 300 nm long, 1.5 nm wide molecule composed of the triple-helical molecules staggered into fibrils. The ordered packing of the triple helices allows for the collagen fibers to be durable and resilient. Widespread crosslinking found within collagen molecules lends additional stability to collagen [4].
Collagen Types

Currently, 28 unique types of collagen have been identified, however types I, II, III, and V are the main types that comprise the collagen in bone, cartilage, tendon, skin, and muscle and type I collagen is the most abundant found in animals, accounting for 90% of the collagen found in humans. The various types of collagen can be organized into subcategories based on the structure they form [5].
Type I collagen is abundant in skin, tendon, ligaments, bones, and teeth. Type II collagen has smaller diameter fibrils than type I and is found mostly in the cartilage and the eye. Type III collagen is found in the skin and blood vessels. Type V is found in cell surfaces and hair [5].
Of the many types of collagen, only fibrous collagen molecules, types I, II, III, and V, have seen use in the production of biomaterials, with type I seeing the most usage [6]. The unique properties of these collagen molecules are due to the ability of the triple helices to form compact fibrils which then aggregate into durable fibers.
Motivation
Collagen of various types forms the foundation of many tissues found in living organisms. Part of collagen’s appeal as a biomaterial is its natural abundance and that it can be extracted from the tissue of almost any animal. Collagen from bovine skin, porcine skin, and rat tail are most commonly used for biomedical applications [6].
Due to its compact structure, collagen fibers have remarkable mechanical properties and stability. In addition, collagen’s structure make the protein resistant to proteolysis and enzymatic degradation at neutral pH. However, its degradation in vivo can be manipulated via pH change making it an easily controllable property based on the application. Collagen based materials can often be absorbed into the human body after their purpose has been completed [1]. Collagen has also been used as a biomaterial due to low toxicity and immunogenic reactions. DeLustro et al. investigated the immunogenicity of various collagen products and found that the dominant immunological response was due to the small quantities of non-collagenous proteins present [7]. This can be attributed to the similarity in amino acid sequence in collagen among species. Animal collagen in the form of sutures or hemostatic agents is considered safe and making it suitable for use as a biomaterial.
At first, collagen was believed to have purely a role in supporting structure in biological systems; however, researchers discovered that collagen has a role in many cellular functions including growth, differentiation, and migration [6]. It has been demonstrated that collagen can enhance the growth of different cell types, and type I collagen substrates have been used for the culture of various cells [8]. Hugues et al. showed that adhesion to collagen molecules is necessary in order for platelet activation and enhance wound healing [9].
High biocompatibility and biodegradability make collagen ideal for use in biomedical applications. The main biomedical applications of collagen are for wound cover dressings, osteogenic and bone filling materials, antithrombogenic surfaces, and immobilization of therapeutic enzymes. More recent work has seen collagen explored as a drug delivery carrier. Collagen-based materials have also seen use as implants in periodontal applications and in cosmetic surgeries. Moreover, collagen is biodegradable, non-toxic and readily absorbed[1].
History
1881 – collagen first used as a biomaterial by Joseph Lister and William Macewen who both independently discovered a biodegradable material appropriate for use in sutures known as “catgut”, a collagen rich material extracted from the small intestine of sheep [6].
1938 – Collagen structure suggested by Astbury comprising a mix of trans and cis peptide units [10].
1955 – Ramachandran et al. proposed a correct triple-helical model for collagen which had one-third of its amino acid residues as glycine [11].
1956 – Ehrmann and Gey demonstrate that collagen can enhance the growth of different cell types [12]. 1960 – Hugues et al. show that collagen promotes platelet production and wound healing [9].
1973 – Chvapil et al. show collagen powders exhibit excellent adhesion to wound [13].
1982 – Protocol for preparation of purified, soluble collagen described by Miller and Rhodes [14].
1987 – Doillon et al. demonstrate collagen substrates promote growth, differentiation, and migration of cells [15].
1998 – Wang et al. show collagen-based membranes have application as barriers that inhibit migration of epithelial cells and encourage wound healing [16].
Applications
Medical Uses

Collagen based devices have seen application in a variety of ophthalmological practices. Collagen shields were designed as bandage contact lenses, which are implanted for protection following surgery and gradually dissolve in the eye. These can be for purely protective purposes or can also be used to administer drugs to the eye. The shield protects the healing cornea from agitation due to the blinking action of eyelids. Drugs can be immobilized within the collagen matrix and are released as the shield dissolves. Collagen inserts are also commercially available as a method of drug delivery to the eye. These are either cut from films or as molded rods with drugs loaded inside them. Suspended collagen particles have also been explored as a potential drug delivery method for the eye [17].

Collagen sponges have seen use as wound dressings for severe burns, pressure sores, donor sites, and leg ulcers. Collagen sponges have been combined with other materials in composites in order to allow for more resilience and fluid binding capacity. Due to collagen’s hydrophilicity, collagen sponges can adhere to soft tissue and allow a surface for new tissue growth. In addition to enhancing wound healing, collagen sponges can also be used for the delivery of antibiotics to wounds [1]. While most medical functions of collagen involve its application as a solid, injection of soluble collagen has been used in order to aid the repair of dermatological defects. Injectable collagen has also been used to deliver growth factors and enhance cellular regeneration and tissue repair. Collagen hydrogels provide a large, uniform surface area which allow them to serve as a drug delivery system [3].
Films made from hydrolyzed collagen have been used as a tissue adhesive for sutures due to its chemical similarity to connective tissue and its tissue fluid-binding properties. Collagen films also show slow, easily controllable release of encapsulated drugs [17].
The most widespread role of collagen biomaterials is in wound dressings and sutures. Collagen-based wound dressings are easily molded. Surgical adhesives have been synthesized from porcine collagen to prevent air from leaking out of lungs. Collagen sutures are commonly derived from collagen of the bovine small intestine. The material is then treated with an aldehyde solution which imparts resistance to enzymatic degradation on the suture [6].
Dentistry
Due to the fact that collagen can enhance wound healing via clot formation and stabilization, it has seen use in healing applications following dental procedures. Experiments conducted by Pitaru et al. indicate that type I collagen is capable of supporting regeneration of periodontal tissues [18]. A multitude of collagen based products are available for the control of bleeding post-dental surgery. Products are soft, white, sponge-like structures that when applied to a bleeding surface rapidly absorb blood and create an artificial clot. They achieve hemostasis within 2 to 5 minutes and if left in place will completely resorb within 56 days. Collagen films have been used as a replacement for sutures due to its chemical resemblance to connective tissue and the fact it does not hinder the healing process. Collagen-based scaffolds have been used in order to facilitate the growth of endothelial cells. [19] Collagen membranes have also been used as barriers to the migration of epithelial cells to encourage healing by providing room for regenerative cells in guided tissue regeneration (GTR) processes. A variety of collagen membranes of type I and type III are available commercially for GTR [16]. Collagen films and sponges are both used as temporary and permanent wound dressings in dental applications. The three dimensional structure of sponges composed of large pores allow for cell migration into them and tissue growth. Crosslinked collagen films on a methacrylate substrate are another form of collagen used as oral wound dressings [19].

Tissue Engineering
![A TEM image of a porous collagen sponge [6]](https://s3-us-west-2.amazonaws.com/oww-files-thumb/e/e0/Porouscollagen.PNG/300px-Porouscollagen.PNG)
Porous collagen sponges have been used in the growth of cell cultures for both tissue engineering and direct implants. These have been used to promote formation of cartilage, abdominal walls, and axons. These sponges can be made from type I collagen or synthetic collagen [3].
Collagen scaffolds are used to study cell migration, proliferation, and differentiation. They have also been used to study in vitro how cells behave in complex environments. Collagen scaffolds can be produced using a variety of methods in order to allow access to cell membranes, model the nervous system, or allow for the investigation between cancer cells and other cell types in a 3D environment. Scaffold can also be hardened via mineralization in order to allow for their use in bone and cartilage regeneration [3].
Challenges
In most collagen extraction and purification methods, intramolecular crosslinking is lost which results in decreased strength of the material. In order to obtain collagen of additional strength, additional processing steps must be undergone in order to restore cross-links [6].
The presence of collagen can also cause changes in cell growth or movement within biological tissues. As cells can interact with collagen easily, cells can pull and reorganize collagen fibers resulting in shrinkage or loss of shape in collagen scaffolds [3].
Collagen molecules can occasionally cause an immune response when they are foreign to a host organism. This immune response is due to the production of antibodies to the foreign collagen and may cause organ damage. Studies have shown that collagen types III, V, and VI lead to a much larger antibody response than type I collagen. There are three locations on the collagen molecule which can be targeted for antibody creation, on the terminal non-helical telopeptide ends of the molecule, and a central point known as the helical (A-) determinant. As such, these areas must be either removed or hidden in collagen processing before it is suitable for implantation or injection. For one commercially available collagen implant, Zyderm®, 3% of patients experienced elevated anti-implant antibody levels, while another 2% reacted in response to a second injection [17]. These patients experienced localized inflammation as a result of their implants.
Future Directions
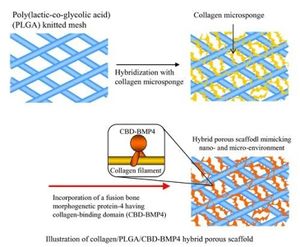
Biomaterial research as a whole has moved from focusing on singular materials to composite materials in order to create biomaterials with desirable properties of both. In particular, collagen and hydroxyapatite composite materials are being investigated due to the high mechanical properties of hydroxyapatite and the cellular interactions of collagen. Another research focus is on the physical properties of collagen in bones and whether they can be manipulated to increase fracture resistance [20].
References
[1] Khan, R.; Khan, M. Use Of Collagen as a Biomaterial: An Update. Journal of Indian Society of Periodontology J Indian Soc Periodontol. 2013, 17, 539.
[2] Lullo, G. A. D.; Sweeney, S. M.; Korkko, J.; Ala-Kokko, L.; Antonio, J. D. S. Mapping The Ligand-Binding Sites and Disease-Associated Mutations on the Most Abundant Protein in the Human, Type I Collagen. Journal of Biological Chemistry. 2001, 277, 4223–4231.
[3] Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-Based Biomaterials For Tissue Engineering Applications. Materials. 2010, 3, 1863–1887.
[4] Lodish H, Berk A, Zipursky SL, et al. Molecular Cell Biology. 4th edition. New York: W. H. Freeman; 2000. Section 22.3, Collagen: The Fibrous Proteins of the Matrix. Available from: http://www.ncbi.nlm.nih.gov/books/NBK21582/
[5] Ramshaw, J. A. M.; Glattauer, V. Collagen-Based Biomaterials. In Biotechnology And Genetic Engineering Reviews; Werkmeister, J. A., Ed.; December 1995; Intercept Ltd: Andover, 1995; Vol. 13, pp. 335–382.
[6] Chattopadhyay, S.; Raines, R. T. Review Collagen-Based Biomaterials for Wound Healing. Biopolymers. 2014, 101, 821–833.
[7] Delustro, F.; Condell, R. A.; Nguyen, M. A.; Mcpherson, J. M. A Comparative Study of the Biologic and Immunologic Response to Medical Devices Derived from Dermal Collagen. Journal of Biomedical Materials Research J. Biomed. Mater. Res. 1986, 20, 109–120.
[8] Kleinman, H. K. Role Of Collagenous Matrices in the Adhesion and Growth of Cells. The Journal of Cell Biology. 1981, 88, 473–485.
[9] Hugues, J. Collagen Platelet Joining. Comptes Rendus de Seances de la Societe de Biologie et deses Filiales 1960, 154, 866-868
[10] Astbury, W. T. The Fourth Spiers Memorial Lecture. X-Ray Adventures among the Proteins. Trans. Faraday Soc. Transactions of the Faraday Society. 1938, 34, 378.
[11] Ramachandran, G. N., and Ambady, G. K. Elements of the helical structure of collagen. Curr. Sci. 1955, 23, 349-350
[12] Ehrmann, H., Gey, R. Section Of Biological And Medical Sciences: Cohesion Of Collagen Fibers In Rat Tail Tendon: Effects Of Growth, Aging And Endocrine Factors,. Transactions of the New York Academy of Sciences. 1969, 31, 855–867.
[13] Chvapil, M.; Kronenthal, R. L.; Winkle, W. V. Medical And Surgical Applications of Collagen. International Review of Connective Tissue Research. 1973, 1–61.
[14] Miller, E. J.; Rhodes, R. K. [2] Preparation And Characterization of the Different Types of Collagen. Methods in Enzymology Structural and Contractile Proteins Part A: Extracellular Matrix. 1982, 33–64.
[15] Doillon, C. J.; Silver, F. H.; Berg, R. A. Fibroblast Growth on a Porous Collagen Sponge Containing Hyaluronic Acid and Fibronectin. Biomaterials. 1987, 8, 195–200.
[16] Wang, H.-L.; Modarressi, M.; Fu, J.-H. Utilizing Collagen Membranes for Guided Tissue Regeneration-Based Root Coverage. Periodontology 2000. 2012, 59, 140–157.
[17] Friess, W. Collagen In Drug Delivery and Tissue Engineering. Advanced Drug Delivery Reviews. 2003, 55, 1529–1530.
[18] Pitaru, S.; Tal, H.; Soldinger, M.; Grosskopf, A.; Noff, M. Partial Regeneration Of Periodontal Tissues Using Collagen Barriers. Journal of Periodontology. 1988, 59, 380–386.
[19] Mahesh, L..; Kurtzman, G.M.; Shukla, S. Regeneration in Periodontics: Collagen – A Review of Its Properties and Applications in Dentistry. Compendium. 2015, 36, 5-7
[20] NIH grant will fund IUPUI research into collagen's role in bone fracture resistance : Newscenter : IUPUI. http://news.iupui.edu/releases/2015/07/research-collagen-bone-fracture.shtml
--Hieu La 02:02, 2 February 2016 (EST)
