CH391L/S12/Unnatural Amino Acids
Unnatural Amino Acids
Introduction
The genetic code for the translation of RNA into protein is one of the most ancient and universal innovations in the evolution of life on earth. Nearly all life forms use the same redundant code for the incorporation of the 20 canonical amino acids into proteins. In two unique exceptions, selenocysteine and pyrrolysine, stop codons have been retooled to code for a 21st amino acid. [1][2] Expansion of the genetic code to include noncanonical, or unnatural amino acids (UAAs) holds promise for improving and diversifying protein function, generating proteins that normally would require postranslational modification, and the study of the genetic code itself. Technology for the creation of proteins bearing UAAs has progressed steadily over the last ~30 years, including both in vitro and in vivo methods.
In vitro Synthesis


Solid-phase Synthesis and Chemical Ligation
Solid-phase peptide synthesis (SPPS) was developed by Bruce Merrifield in the early '60s, for which he received the Nobel Prize in 1984. In this method, the C-terminal amino acid is anchored via a linker to an insoluble support. Both the N-terminus and the side-chain are protected from reaction. The N-terminus is typically protected by Boc or Fmoc groups, and the side-chains can be protected by a variety of groups. In the first step, the N-terminus is deprotected. The next desired amino acid in the chain is added to the column. This process of deprotection and addition is repeated until the chains is completed. The side-chains are then deprotected, and the completed peptide is eluted from the column. This method is useful for producing peptides of up to ~50 amino acids.
Native chemical ligation is used to produce larger peptides and full proteins. This method requires uniquely reactive functionalities incorporated into each peptide at the N- and C-terminus, and allows the use of unprotected peptide segments. In one method of native chemical ligation, the thiolate group of an N-terminal cysteine residue peptide attacks the C-terminal thioester of a second unprotected peptide. This reversible transthioesterification step is chemoselective and regioselective and leads to form a thioester intermediate. This intermediate rearranges irreversibly by an intramolecular S,N-acyl shift that results in the formation of a native peptide bond at the ligation site. This method may be repeated to make long peptides.[4]
E. coli Strains
Cloning
Protein Expression
Orthogonality
Genome Reduction
Systematic Genome Reduction
One natural approach to engineering strains with a reduced genome is to systematically identify and delete regions of the genome not necessary for host cell survival. Posfai et al. created the MDS strains (multiple deletion strains) by aligning the genomes of multiple genomes of E. coli, identifying regions which were absent in multiple strains, and deleting them via Lambda Red recombination. All IS elements were removed as well, lowering the mutation rate and increasing the stability of genetic constructs introduced into the cell. The strain had comparable growth rate compared to wild type.[5][6]
Ara et al. constructed a minimal version of the B. subtilis genome in 2007. [7] This strain had slightly decreased growth rate compared to wild-type, but displayed normal morphology and similar protein production capabilities.
Selection for Reduced Genome
Long-term evolution of strains under the correct conditions could select for a genome of minimal size. Such conditions may include growth in rich media lacking sugars to favor the loss of biosynthetic pathways or sugar metabolism operons, growth in structured environments which favor a smaller cell, or growth under other conditions which favor the loss of unnecessary genes.

Minimal Genome Synthesis
Another approach is the synthesis of a minimal, designed genome from scratch using DNA synthesis technology and the transformation of this genome into cells to create a viable, novel, synthetic organism. This approach can be viewed as forward engineering of a novel organism, but would likely be informed by studies which determine the minimal set of genes necessary for a living organism.
Mycoplasma mycoides Synthesis
Gibson et al synthesized the first artificial cell by generating the Mycoplasma mycoides genome from digitized genome information and transforming it into Mycoplasma capricolum cells devoid of genomic information. These cells were capable of continuous self-replication and were identified by "watermarks" inserted in the genome. This technology could be utilized in the future to create cells with novel and useful properties from scratch.[8][9]
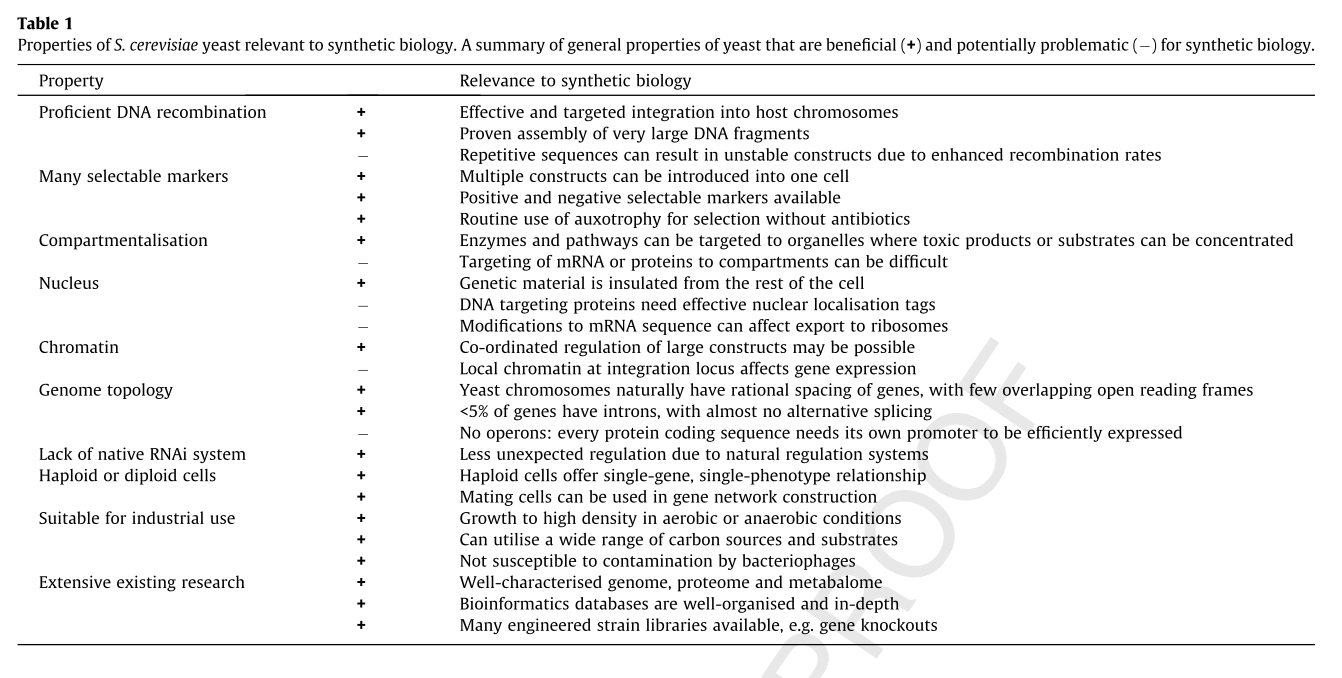
Yeast as a Model Organism
The following table summarizes some of the advantages and disadvantages of using yeast as a model organism for synthetic biology. [10][2]
References
- Longstaff DG, Larue RC, Faust JE, Mahapatra A, Zhang L, Green-Church KB, and Krzycki JA. A natural genetic code expansion cassette enables transmissible biosynthesis and genetic encoding of pyrrolysine. Proc Natl Acad Sci U S A. 2007 Jan 16;104(3):1021-6. DOI:10.1073/pnas.0610294104 |
- Böck A, Forchhammer K, Heider J, and Baron C. Selenoprotein synthesis: an expansion of the genetic code. Trends Biochem Sci. 1991 Dec;16(12):463-7. DOI:10.1016/0968-0004(91)90180-4 |
- Dawson PE and Kent SB. Synthesis of native proteins by chemical ligation. Annu Rev Biochem. 2000;69:923-60. DOI:10.1146/annurev.biochem.69.1.923 |
- Schnölzer M and Kent SB. Constructing proteins by dovetailing unprotected synthetic peptides: backbone-engineered HIV protease. Science. 1992 Apr 10;256(5054):221-5. DOI:10.1126/science.1566069 |