CH391L/S12/Artemisinic Acid Engineering
- NOTE* Page is still under construction. As long as this note appears, assume information presented on this page is incomplete and unreliable.
Artemisia

Artemisia is a genus that encompass a broad range of plants commonly known as sagebrush, sagewort, and wormwood. The family, which derives its name from the either from the Greek goddess Artemis or Artemisia, the wife of Mausolus, includes many famous plants. Artemisia absinthium is used in the production of Absinthe, and Shakespeare employed wormwood as a cure for the love potion in Midsummer's Night Dream. One species of Artemisia, known as Artemsia annua, has been used for thousands of years in traditional Chinese medicines to cure headaches.
This last species is of particular interest to synthetic biologists, due to its ability to produce Artemisinin. As a result, much of the species' genes have been characterized and submitted to the Gene Bank.
Artemisinin
Aretmisinin, or Qinghaosu, is a chemical compound produced naturally by the plant Artemsia annua. It is a sesquiterpene lactone, a type of terpene derived from 3 isoprene units and a lactone ring. The compound is unique, as it contains an oxygenated cyclic ring with a peroxide bridge, and contains no Nitrogen atoms. It was first derived from Artemsia annua in 1971 by Chinese chemists interested in observing the plant's supposed use in curing headaches [1]. In response to the Chinese government's push to develop new malaria cures, malaria patients were treated with this compound[2]. Fortunately, it was determined to be very effective at combating common malaria strains, including those resistant to the traditional antimalarial drug chloroquine. Currently artemisinin and its derivatives are employed to combat the malaria strain P. falciparum.
In 2011, the highly prestigious Lasker DeBakey Clinical Research Award was given to Youyou Tu,the principle investigator from the Institute of Chinese Meteria Medica, China Academy of Chinese Medical Sciences whose team first discovered artemisinin in 1969. Her team was engaged in project 523, an attempt to screen over 2,000 traditional Chinese medicinal cures for potential use to combat malaria in the tropics of Vietnam [3]. Later tests in humans infected with malaria indicated that the drug worked so well within hours after application, that many individuals would discontinue use. Unfortunately, the malaria symptoms would eventually return, requiring new application of artemisinin[3]. The discovery of artemisinin by Dr. Tu has been talked about as a possible nominee for the 2012 Nobel Prize in medicine.
Artemisinin posses antimalarial capabilities due to its trioxane ring structure. It is capable of killing the malaria parasite while infecting human red blood cells, and even proved effective on strains resistant to commonly used antimalarial drugs. Unfortunately, the ketone group on the bottom of artemisinin, shown below, imparts negative characteristics. In its native form, artemisinin has low solubility in both water and oil. This makes the compound difficult to produce and distribute, and led to the development of its multiple derivatives shown below. Note the expansion of the ketone group on the bottom of artemisinin in successive derivatives, which contribute to their increased solubility and potency[2].
Chloroquine
Previously, one of the most widely used antimalarial drugs produced was chloroquine. While successful at combating malaria, chloroquine exhibits multiple adverse side effects, most notably Pruritus. Pruritus is a particularly uncomfortable form of itching sensation that dark-skinned individuals are more susceptible to, due to their increased melanocytes and the pathway in which chloroquine binds to these melanocytes[4]. This condition can be so painful that multiple drugs must be administered in unison with chloroquine in order to negate its detrimental side-effects. Considering that malaria generally plagues the poorest 20% of the world's population, mainly located in countries in Africa, the cost-effectiveness of this procedure can make it impractical to implement.
Production of Artemisinic Acid
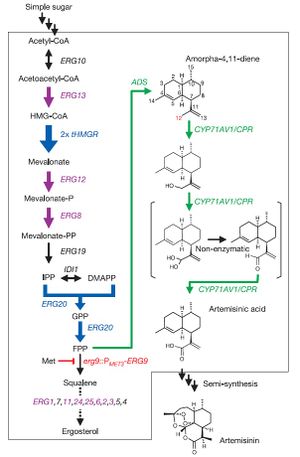
In a famous paper released by Ro et al., Saccharomyces cerevisiae was engineered to produce artemisinic acid from simple sugars. This involved metabolic engineering of the mevalonate pathway, naturally present in S. cerevisiae, to introduce a three-step pathway that converts amorpha-4,11-diene into artemisinic acid.
The first step of this process involved engineering the yeasts' natural mevalonate pathway to produce increased levels of farnesyl pyrophosphate (FPP). 3-hydroxy-3-methyl-glutaryl-coenzyme A reductase (tHMGR) was over-expressed in S. cerevisiae by introducing nother copy of the gene encoding for this enzyme, leading to increased mevalonate production by fivefold. Additionally, the mutant allele upc2-1 was introduced, leading to moderate over-expression of various ERG genes responsible for the production of enzymes in the mevalonate pathway.
S. cerevisiae naturally employes mevalonate to produce sterols. Ro et al. therefore introduced a methionine-repressible promoter to down-regulate the enzyme-encoding gene ERG9, responsible for the conversion of mevalonate into squalene.
Natively, S. cerevisiae contains no pathway to produce any form of artemisinin or its precursors. Therefore, Ro et al. isolated the genes encoding amorphadiene synthase (ADS) from Artemsia annua, and introduced them into S. cerevisiae. Alone, this step led to low levels of amorphadiene. However, combined with the steps mentioned above, significantly higher levels of amorphadiene were achieved.
Finally, Ro et al. isolated the genes responsible for converting amorphadiene to artemisininc acid in Artemsia annua and introduced them into S. cerevisiae. First, a genomic analysis of plants in the Asteraceae family was performed to determine common ancestor enzymes. It was determined that cytochrome P450 monooxygenase from sunflower and lettuce were compatible, and P450 gene fragments were obtained from the Asteraceae EST-database. A cDNA library of P450 fragments was created using random primers specific to CYP71 Asteraceae subfamily, and BLAST analysis was employed to determine the conservation of P450 gene across the entire Asteraceae family. The BLAST analysis determined that the P450 gene was an acceptable candidate for introduction into S. cerevisiae. The isolated P450 cDNA encoding for CYP71AV1 was then incorporated into S. cerevisiae. For stability of this CYP71AV1 enzyme, cytochrome P450 oxidoreductase (CPR) was also isolated from Artemsia annua and introduced into S. cerevisiae. This allowed for an effective 3-step oxidation pathway to convert amorphadiene into artemisinic acid.
In vivo tests were then performed to determine whether this engineered S. cerevisiae was actually capable of producing artemisinic acid. Results of growth tests indicated that high levels of artemisinic acid were indeed present, and actually attached to the outer cell surface of the yeast cells. This allowed for easy recovery of plentiful amounts of artemisinic acid.
The results of Ro et al.'s work was the ability to rapidly produce high levels of artemisinic acid. The yeast engineered in this study were capable of producing 4.5% dry weight of artemisinic acid, roughly 3.5 times the productive levels of Artemsia annua. Additionally, the S. cerevisiae completes a full growth cycle in roughly one week, while Artemsia annua requires multiple months to grow completely.
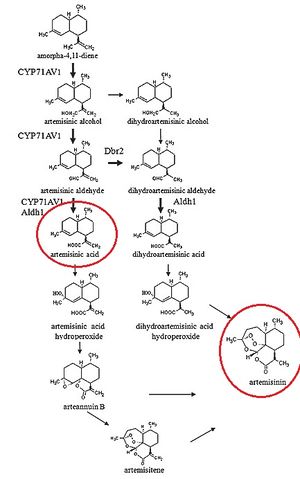
It is worthwhile to note that the process by which artemisinic acid is converted into artemisinin is currently under debate, as "...how artemisinic acid transforms into artemisinin is still under investigation" [6]. However, multiple processes by which artemisinic acid can be converted into artemisinin have been suggested [7]. It has been shown that the conversion of artemisininc acid into artemisinin appears to be the rate-limiting step in the production of artemisinin in Artemsia annua[8], suggesting that improvements can be made in this mechanism.
Relation to iGEM
There has been no incidence of previous iGEM teams investigating artemisinin or any of its precursors. Many iGEM teams have referenced Ro et al., but only as an example of pathway engineering, or in order to discuss ethics and synthetic biology's potential to positively benefit society. No parts in the parts registry deals with artemisinin either. Only one [set of parts] mentions a protein, Granulysin, which can potentially be used to fight malaria. Investigation of further applications for artemisinin and its derivatives could serve as a novel area of research for future iGEM teams.
References
- Klayman DL. Qinghaosu (artemisinin): an antimalarial drug from China. Science. 1985 May 31;228(4703):1049-55. DOI:10.1126/science.3887571 |
Early review article that details the development of artemisinin and its derivatives for antimalarial drugs.
- Brown GD. The biosynthesis of artemisinin (Qinghaosu) and the phytochemistry of Artemisia annua L. (Qinghao). Molecules. 2010 Oct 28;15(11):7603-98. DOI:10.3390/molecules15117603 |
Relatively new review article on artemisinic acid and its derivatives.
- Miller LH and Su X. Artemisinin: discovery from the Chinese herbal garden. Cell. 2011 Sep 16;146(6):855-8. DOI:10.1016/j.cell.2011.08.024 |
Follows the discovery of artemisinin by Youyou Tu
- Aghahowa SE, Obianwu HO, Isah AO, and Arhewoh IM. Chloroquine-induced Pruritus. Indian J Pharm Sci. 2010 May;72(3):283-9. DOI:10.4103/0250-474X.70471 |
Study from the University of Benin that explores the detremential side-effects of chloroquine, particularly pruitus.
- Ro DK, Paradise EM, Ouellet M, Fisher KJ, Newman KL, Ndungu JM, Ho KA, Eachus RA, Ham TS, Kirby J, Chang MC, Withers ST, Shiba Y, Sarpong R, and Keasling JD. Production of the antimalarial drug precursor artemisinic acid in engineered yeast. Nature. 2006 Apr 13;440(7086):940-3. DOI:10.1038/nature04640 |
Widely referenced Nature article from the Keasling lab in UC Berkley in which the researchers were able to produce artemisinic acid in S. cerevisiae at levels over 100 times that produced naturally in Artemisia annua.
- Wen W and Yu R. Artemisinin biosynthesis and its regulatory enzymes: Progress and perspective. Pharmacogn Rev. 2011 Jul;5(10):189-94. DOI:10.4103/0973-7847.91118 |
Reviews the biosynthetic pathways for the synthesis of artemisinin in Artemisia annua.
-
Acton N and Roth JR. DOI: 10.1021/jo00039a020
Suggests multiple possible pathways in which artemisinin might be produced from artemisinic acid.
-
Zhang YS, Ye HC, Liu BY, Wang H and Li GF. DOI: 10.1007/s11183-005-0009-6
Notes that the rate-limiting step in the chemical reaction for artemisinin production is the conversion from artemsininc acid.
