Robert Langer

Introduction
Robert Langer is perhaps the most notable chemical engineer to have dabbled in biology [1-5]. In 1974 he graduated with a PhD in chemical engineering and instead of joining his peers in the oil industry he pursued work to benefit human health. His postdoctoral studies began by joining Judah Folkman's lab group at the Boston Children's Hospital.
"It was my first exposure to biology and Judah was very visionary, so it was a great experience for me" [1].
Judah Folkman (1933-2008) was a prominent cancer researcher who is largely credited for his work on angiogenesis--in short the growth of blood vessels [4]. Under Folkman's mentorship Langer set out to find inhibitors of angiogenesis [4]. Langer's unique thinking landed him quite the discovery. He observed that cartilage (ex. from cows) contains blood vessels in an embryonic state yet as the tissue matures it loses them. He lysed mature cartilage and tested whether that protein solution would inhibit in vitro blood vessel growth--it did. Towards the end of his post-doc, he moved on to discover a method to deliver large molecules, in a controlled manner, to different parts of the body using plastic scaffolds. His work with Folkman launched his career in biomedical and tissue engineering. Below are brief summaries of his most significant (by times cited) publications [according to the Web of Science Database].
"Tissue Engineering," Science (1993)
"Tissue Engineering" Science; May 14, 1993; 260, 5110; ProQuest Education Journals pg. 920
Langer's most referenced paper, cited over 3400 times, explores what tissue engineering is and it's potential applications. His paper breaks down into three body paragraphs, each discussing the progress made in a given germ layer (ectoderm, endoderm, and mesoderm).
Introduction:
- Tissue and organ failure have a significant impact to public health
- Donor shortage problems preclude extensive use of transplantation
- Mechanical devices are not yet capable to serve as organ replacements
- Tissue engineering is:
"... an interdisciplinary field that applies the principles of engineering and the life sciences toward the development of biological substitutes that restore, maintain, or improve tissue function."
- General strategies employed by tissue engineering
- Isolating cells
- Inducing tissues with signaling molecules
- Open and closed cell scaffolds (cells on or inside of matrices)
Ectoderm:
Nervous System
- Transplantation of cells to produce a given molecule that is made in insufficent quantities--causing a disorder (ex. encapsulating and transplanting dopamine-producing cells to reverse Parkinson's)
- Enhancing nerve regeneration with polymeric guides
Cornea
- Artificial corneas which adhere epithelial cells and have appropriate permeability to light, nutrients, and signaling molecules. Animal studies have begun with epithelial cell-seeded polyvinyl alcohol hydrogels.
Skin
- Tissue transplants to treat burn wounds, ulcers and other skin wounds
- Composite skins made of an outer silicone layer (prevents fluid flux), which is later replaced with a thin epidermal graft, and an inner chondroitin-sulfate/collagen layer. The chondroitin-sulfate/collagen layer is biocompatible--connective tissue grows with it.
- Alternatively, skin grafts can come from in vitro cultivated keratinocytes grown on a feeder layer of fibroblasts. These grafts can cover very large wounds, however they take 3-4 weeks to expand the epidermal cells.
- Alternatively, seed dermal fibroblasts in a degradable polyglycolic acid mesh or collagen gel. Since the gels are degradable, a graft vascularizes (very useful for deep injuries) when transplanted and allows the formation of organized tissue. Clinical trials have begun
Endoderm:
Liver
- Restoring the liver's bilirubin and urea metabolization capability by transplanting microcapsule-encapsulated hepatocytes
- Extracoroperal systems which contain hepatocytes
Pancreas
- Transplanting encapsulated pancreatic islets, seeded polymer grafts, or hollow fibers with immobilized cells to restore function
Mesoderm:
Cartilage, bone, and muscle
- Create synthetic cartilage from collagen-glycosaminolycan templates and chondrocytes
- Implanting demineralized bone powder (DBP), delivering bone morpogenic proteins (BMP), or growth factors (ex. TGF-beta) to stimulate bone formation. Use recombinant technology to synthesize sufficent quantities of such molecules.
Heart
- Auto-transplantation of in vitro expanded satellite skeletal muscle to regenerate damaged heart muscle.
- Creating vascular grafts out of polytetrafluoroethylene or Dacron. Minimizing clotting and scarring problems by making these grafts inert (heparin coating) or lining them with endothelial cells.
- Creating replacement red blood cells (RBCs)--oxygen transporters--by encapsulating hemoglobin or perfluorocarbons (PFCs). Hemoglobin and PFCs have high oxygen affinity
Conclusion:
- Cell sourcing and preservation are common challenges to most tissue engineering solutions
- Polymer processing needs to be more reproducible and controlled before clinical applications can proceed
- Tissue engineering may solve donor shortage and cost issues arising from today's transplantation and reconstruction therapies.
- Tissue engineering will require an interdisciplinary research effort
"Drug delivery and targeting" Nature (1998)
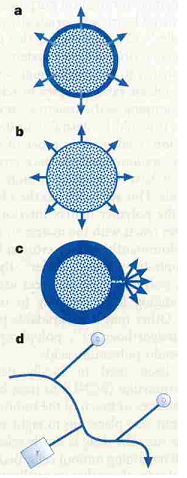
Langer's 1998 paper has been cited nearly 900 times. In this publication he presents strategies to encapsulate or attach drugs to a polymer and "intelligently" deliver them.
Introduction
- Drug delivery is important because therapeutics can have cytotoxic or at least harmful effects to non-target tissue.
- Targeting drugs can increase the dosage efficiency
- less of the drug may be necessary quantity
- directed delivery can speed up the time it takes for a drug to reach its target.This is especially attractive for drugs with short half-lives
Polymer-Based Systems
- Drugs can be released by diffusion or cleavage from their scaffolds/carriers or by degradation or swelling of polymer.
- Figure 3 shows and describes various drug-delivery strategies
- Norplant silicone capsules have been used to slowly release contraceptives via diffusion through the polymer
- Stability of the polymer being used and its porosity or chief parameters of concern
- designing polymers with outer, hydrophobic monomers to prevent water from degrading the eroding the matrix
- Polymer can be functionalized to be bioadhesive (RGD, hydrogen bonding, etc.) thereby allowing for tunable residence time
- Drug binding, as opposed to encapsulation and then release, give drugs different half lives and immunogenicity.
Targeting Applications
- Passive targeting strategies involve flowing a therapeutic through the entire body, yet having the drug accumulate only in target tissue.
- Small-molecule anticancer drugs can be linked to a large, inert polymer. Since only tumor tissue has "leaky" microvasculatures (capillaries), the drugs accumulate primarily in cancerous tissue. ex. N-(2-hydroxypropyl) methacrylamide conjugated to doxorubicin accumulates 70x more in mouse tumors rather than normal tissue.
- Active targeting strategies typically couple a polymer with an antibody or carbohydrate that has specific binding with a given tissue/cell type. Ex. immunoconjugates
"Designing materials for biology and medicine," Nature (2004)
With over 900 references "Designing materials for biology and medicine" is Langer's second most referenced paper. He introduces us to what a biomaterial, particular applications, followed by a discussion of design parameters.
What are biomaterials?
- "... substances other than foods or drugs contained in therapeutic or diagnostic systems and... materials composed of biologically derived components..."
- Wooden teeth and glass eyes were the earliest biomaterials to be used (over 2,000 years ago)
- biocompatibility and degradation are critical issues in development
Synthetic Tissue Replacements
- Biological macromolecules and artificial proteins can be synthesized via recombinant DNA technology
- Novel proteins can be fabricated with controlled mechanical properties of protein by expression of artificial DNA or modifications of existing genes.
- Biomimetic polymers are coupled with biological markers (ex. amino acid sequences) thus giving these tailorable synthetic polymers the "informational and multifunctional character[s] of natural materials"
- RGD sequences that permit control of cell adhesion on a given substrate
- Materials can be made that change in shape or surface property due to pH, light, or temperature changes.
- pH changes (for example the gastrointestinal tract) trigger gel swelling of allowing for the selective release of encapsulated proteins to the basic intestine
- Shape-memory materials could be useful in the design of synthetic viral vectors which need to condense DNA, enter a cell, and release their contents to the nucleus.
New Applications
- Diagnostic screens can be developed out of biomaterilals displaying complementary proteins domains or DNA sequences
- Protein arrays could allow for doctors to quickly identify protein levels in cells and tissues. However, they are especially challenging to design because of diverse charge and hydrophobic characters of proteins, in addition to and problems with non-specific binding.
Conclusion
- Biomaterials development will need to tackle targeting and biocompatability problems
- Materials capable of sensing and detecting biochemical signals are of great interest
References
[1] Career Path: Robert Langer, Nature Reviews: Drug Discovery. Volume 4 (2005)
[2] Langer Lab Website (2012) <http://web.mit.edu/langerlab/>
[3] Faculty Sketches: Robert S. Langer Junior. (2012) <http://hst.mit.edu/public/people/faculty/facultyBiosketch.jsp?key=Langer>
[4] NOVA Profile: Judah Folkman. (2008) <http://www.pbs.org/wgbh/nova/body/judah-folkman.html>
[5] National Academy of Engineering. "Robert Langer" Committee Profile. <http://www.engineeringchallenges.org/cms/7124/7264.aspx>
[6] Langer R, Vacanti JP. Tissue engineering. Science. 1993;260:920–6
[7] Langer, R.Drug delivery and targeting. Nature 392 (Supp): 5-10, 1998
[8] Langer, R. & Tirrell, D.A. Designing materials for biology and medicine. Nature 428, 487–492 (2004).
