BISC209/S13: Lab9: Difference between revisions
| Line 104: | Line 104: | ||
'''Do:''' | '''Do:''' <BR> | ||
'''Continue to wind down and complete your work on your isolates. '''Discard and clean up any remaining tests and cultures on your isolates. Note that there are 5 bonus points awarded for perfect clean-up. Your instructor will explain the "rules" for obtaining these bonus points. '''<BR> | '''Continue to wind down and complete your work on your isolates. '''Discard and clean up any remaining tests and cultures on your isolates. Note that there are 5 bonus points awarded for perfect clean-up. Your instructor will explain the "rules" for obtaining these bonus points. '''<BR> | ||
Revision as of 18:05, 23 January 2013
LAB 9 Finishing the project!
This is your last wet lab! After you have recorded all your results on a lab Google Doc, you need to freeze your isolates. Once these steps are completed, please discard all cultures. You will receive 5 "clean-up points" the week afterr Lab 10 if we do not find any evidence of your cultures or tests. If we have to discard and clean-up later after you, you will not receive any of those 5 points.
Freezing freshly cultured isolate protocol
Please complete the Google doc prior to freezing your isolate. After you have recorded all your results, follow the instructions found in protocols to freeze your isolates.
Using Mass Spectrometry (MALDI-TOF MS) to Identify and build a library of soil isolates.
Using log phase (24 hour) cultures of your isolates you will learn how the MALDI-TOF Biotyper works to identify your microbes and to begin a "library" of soil organisms. You need freshly grown cultures of your isolates today made 24 hours prior to this lab. If you have one or two very slow growing cultures, you need to allow enough time to have a small but visible colony on your plate.
Using the MALDI-TOF Biotyper to identify your bacterial isolates
NOTE: You will be provided with the instructions for using the MALDI-TOF and Biotyper in lab.
MALDI-TOF Theory
The following MALDI background information is provided courtesy of Bruker Daltronics:
The microflex LT as MALDI TOF
A short survey about technique of Bruker microflex LT MALDI-TOF mass spec.
Glossary
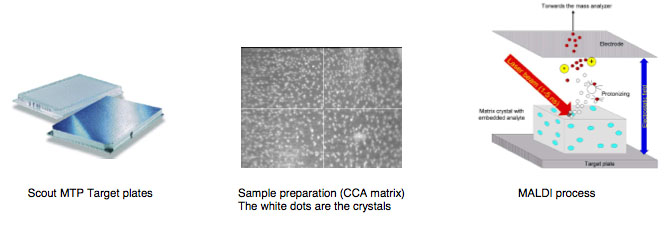
MALDI is the abbreviation for Matrix Assisted Laser Desorption/Ionization
TOF for Time of Flight.
Matrix is a chemical substance which will assist the sample ionization process and must be crystallized in a sample compound on the target surface. Common matrix substances are:
- CCA or HCCA (Alpha-Cyano-4-Hydroxycinnamic Acid)
- SA (Sinapinic Acid)
- DHB (Dihydroxybenzoic Acid)
MALDI-TOF The samples which are measured for MALDI Biotyper application are typically the Proteins of Bacteria, Fungi and Yeast. The mass and distribution information will be used for identification.
By firing with a laser (on microflex a nitrogen uv laser with a wavelength of 337 nm) on the prepared matrix-sample compound the matrix will be vaporized. The sample will be entrained and ionized. The matrix and sample ions will be accelerated in an electrical field as the target plate is under high voltage of up to 20 kV against to an arranged ground potential.
Via the flight time between acceleration and hitting the detector the mass of an ion can be determined (TOF = Time of Flight technique). The complete process needs to take place under vacuum of better than 3*10-6 mbar. As better the vacuum as less collisions
To avoid influences of temperature, mechanical and electrical deviations and special techniques for acceleration the instrument needs to be calibrated before measuring unknown samples. To do this a mixture of known samples will be measured and an assignment between flight time and theoretical mass will be done.
Amongst others the achievable resolution depends on the speed of the detector and the speed of converting the analog signals to digital signals. On microflex a 500 MHz digitizer which achieves a distance from 2 ns between data points (in 2 ns the light covers a distance of just 60 cm) is used. With a special digitizer function provides actually a calculated ‘pseudo’ 2 GHz sampling rate. Mainly influencing variables are also the applied laser energy, the drift distance and the electrical field distribution.
Definition and calculation of mass resolution
The mass resolution for a single peak is calculated by dividing the peak mass by the Full Width at Half Maximum (FWHM). The resolution value can be checked in flexControl and flexAnalysis directly.
Linear mode
The ionized sample will be accelerated in an electrical field between the target plate and the acceleration electrodes. Followed by ‘drifting’ to a field free region and finished by hitting the detector.
Ion acceleration
Continuous extraction:
The first MALDI-TOFs have been used continuous extraction to accelerate ions. Between target plate and ground plate is a static electrical field which accelerates the ions immediately after generating.
Delayed extraction:
Next step was to use a delayed extraction to accelerate ions (pulsed ion extraction - PIE). Between target plate and ground plate a second potential plate (P2) is placed to modify the electrical field. The P2 plate has the same potential as the target plate. So no acceleration will happen after generating the ions. After a delay time, the P2 potential will be decreased and the acceleration starts. The fortune is that the effect of loosing resolving power because of different kinetic energy of equal ions will be reduced. A higher resolution for a small mass range can be observed.
A further advancement could be reached by optimizing the pulse shape applied to the P2 plate. With the now called PAN (panoramic) mode a high resolution over a brought mass range will be reached.
Electrical Lens:
The ion lens is located on ion optic behind the acceleration electrodes and applies a small vectored electrical field to focus the ion cloud for better resolution.
Connections:
The P1 (IS-1) potential (Target plate) is connected to the PIE electronic J497.
The P2 (IS-2) potential is connected to the PIE electronic J495.
The Lens potential is connected to the Lens power supply J480.
Assignment
Graded Assignment: Study for your Lab Practical and submit a complete draft of your final paper'.
Detailed version: Lab Practical tips and Final Paper tips and Writing Guidelines in Resources
Do:
Continue to wind down and complete your work on your isolates. Discard and clean up any remaining tests and cultures on your isolates. Note that there are 5 bonus points awarded for perfect clean-up. Your instructor will explain the "rules" for obtaining these bonus points.
Work on your final paper
.