BISC209/F13: Lab9
LAB 9 Identification of bacteria using MALDI-TOF Mass Spectronomy
Using Matrix Assisted Laser Desorption/Ionization Time of Flight Mass Spectrometry (MALDI-TOF MS) to Identify bacteria
Using log phase (24 hour) cultures of your isolates you will learn how the MALDI-TOF Biotyper works to identify your microbes using a different reference standard than our other ID method (comparison of 16s RNA gene sequences). This method compares particular proteins by mass spectrometry to reference standards. You need freshly grown cultures of your isolates today made 24 hours prior to this lab. If you have one or two very slow growing cultures, you need to allow enough time to have a small but visible colony on your plate.
BACKGROUND:
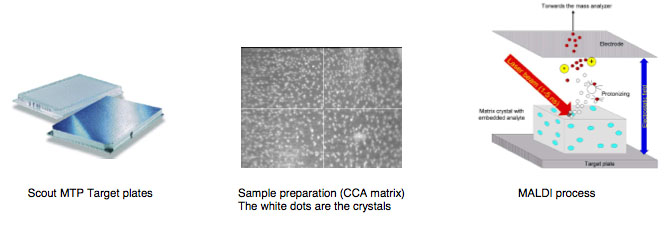
The following MALDI background information is provided courtesy of Bruker Daltronics:
Glossary
MALDI is the abbreviation for Matrix Assisted Laser Desorption/Ionization
TOF for Time of Flight.
Matrix is a chemical substance which will assist the sample ionization process and must be crystallized in a sample compound on the target surface. Common matrix substances are:
- CCA or HCCA (Alpha-Cyano-4-Hydroxycinnamic Acid)
- SA (Sinapinic Acid)
- DHB (Dihydroxybenzoic Acid)
MALDI-TOF The samples which are measured for MALDI Biotyper application are typically the Proteins of Bacteria, Fungi and Yeast. The mass and distribution information will be used for identification.
By firing with a laser (on microflex a nitrogen uv laser with a wavelength of 337 nm) on the prepared matrix-sample compound the matrix will be vaporized. The sample will be entrained and ionized. The matrix and sample ions will be accelerated in an electrical field as the target plate is under high voltage of up to 20 kV against to an arranged ground potential.
Via the flight time between acceleration and hitting the detector the mass of an ion can be determined (TOF = Time of Flight technique). The complete process needs to take place under vacuum of better than 3*10-6 mbar. As better the vacuum as less collisions
To avoid influences of temperature, mechanical and electrical deviations and special techniques for acceleration the instrument needs to be calibrated before measuring unknown samples. To do this a mixture of known samples will be measured and an assignment between flight time and theoretical mass will be done.
The achievable resolution depends on the speed of the detector and the speed of converting the analog signals to digital signals. On microflex a 500 MHz digitizer which achieves a distance from 2 ns between data points (in 2 ns the light covers a distance of just 60 cm) is used. With a special digitizer function provides actually a calculated ‘pseudo’ 2 GHz sampling rate. Mainly influencing variables are also the applied laser energy, the drift distance and the electrical field distribution.
Definition and calculation of mass resolution
The mass resolution for a single peak is calculated by dividing the peak mass by the Full Width at Half Maximum (FWHM). The resolution value can be checked in flexControl and flexAnalysis directly.
Linear mode
The ionized sample will be accelerated in an electrical field between the target plate and the acceleration electrodes. Followed by ‘drifting’ to a field free region and finished by hitting the detector.
Ion acceleration
Continuous extraction:
The first MALDI-TOFs have been used continuous extraction to accelerate ions. Between target plate and ground plate is a static electrical field which accelerates the ions immediately after generating.
Delayed extraction:
Next step was to use a delayed extraction to accelerate ions (pulsed ion extraction - PIE). Between target plate and ground plate a second potential plate (P2) is placed to modify the electrical field. The P2 plate has the same potential as the target plate. So no acceleration will happen after generating the ions. After a delay time, the P2 potential will be decreased and the acceleration starts. The fortune is that the effect of loosing resolving power because of different kinetic energy of equal ions will be reduced. A higher resolution for a small mass range can be observed.
A further advancement could be reached by optimizing the pulse shape applied to the P2 plate. With the now called PAN (panoramic) mode a high resolution over a brought mass range will be reached.
Electrical Lens:
The ion lens is located on ion optic behind the acceleration electrodes and applies a small vectored electrical field to focus the ion cloud for better resolution.
Connections:
The P1 (IS-1) potential (Target plate) is connected to the PIE electronic J497.
The P2 (IS-2) potential is connected to the PIE electronic J495.
The Lens potential is connected to the Lens power supply J480.
Procedure
The Bruker MALDI Biotyper requires organic acid solutions to: 1) prepare the Bacterial Standard (BTS) and HCCA (matrix): 2) complete the Formic Acid Extraction process; and 3) to clean the Polished Steel Target.
Preparing your isolates for MALDI-TOF MS
Formic Acid Isolate Extraction
Principle: In some microorganisms it may be necessary to break down the cell wall and separate the ribosomal proteins prior to spectra analysis.
The Tube Formic Acid Extraction procedure (TE)
- Materials Needed :
- Eppendorf 1.5 μL microfuge tubes
- Eppendorf pipettes/tips
- Microfuge
- Toothpicks (or applicable transfer device)
- Vortex
- Microfuge tube rack
- Bacterial Test Standard (BTS)
- Target
- Ultra-Pure Water, HPLC/MS Grade
- Ethanol, 100% HPLC/MS Grade
- Formic Acid, 70% HPLC/MS Grade
- Acetonitrile, 100 % HPLC/MS Grade
- Fresh Microorganism: overnight growth should be used for routine microorganism identification; slow-growing bacteria may need to cultivate for several days before testing; do not use organisms that have been stored at 40C or lower as this has a negative impact on quality of spectra and reproducibility; storing plates at room temperature for several days is acceptable; different media types and growth temperatures have little effect on results.
1. Add 300 μL of HPLC water to each Eppendorf microfuge tube
2. Transfer a large, single colony of microorganism to the tube (more than one colony may need to be transferred if microorganism is small you want the solution to be opaque/cloudy; chose isolated colonies OR, if using broths, spin and wash (HPLC water) 2-3x to remove media)); Vortex thoroughly (1) minute.
3. Add 900 μL of Ethanol; Vortex thoroughly (1) minute.
4. Tabletop Centrifuge at maximum (13,000 to 15,000 rpm) speed for (2) minutes
5. Decant Ethanol; Tabletop Centrifuge for (2) minutes high
6. Remove ALL excess Ethanol with pipette (tubes may be left at room temperature in the biological hood, or spun more than once, to complete the evaporation process if necessary, all the ethanol must be gone before the next step).
7. Add 50 μL of 70% Formic Acid (if only a small amount (size of pellet) of microorganism is available decrease the Formic Acid volume to 10 μL).
8. Vortex thoroughly 1 min and let stand for 5 minutes.
9. In the chemical hood and WEARING GLOVES, add 50 μL of 100% Acetonitrile (if only a small amount (size of pellet) of microorganism is available decrease the Acetonitrile volume to 10 μL); vortex thoroughly (1) min;
NOTE: the volumes of 70% Formic Acid and Acetonitrile must be equal volumes. (See Formic Acid Extraction Solution 2.)
10.Centrifuge at maximum speed (13,000 to 15,000 rpm) for (2) minute –(continue with: MALDI Target Preparation).
A Bacterial Test Standard (BTS) 10 mg/ml (w/v) is used to calibrate and test software, use fresh on every plate.
The BTS will be provided in lab ready to use.
alpha-Cyano-4-hydroxycinnamic acid (HCCA (MATRIX)) (from Bruker, Fluka, Sigma)
Bruker Product number 255344:.
The reconstituted matrix will be provided in lab ready to use.
MALDI Target Preparation:
Materials Needed:
-
MALDI Biotyper Target
- Bacterial Test Standard (BTS)
- α-Cyano-4-hydroxycinnamic acid HCCA (matrix)
- 2 μL Pipets
- Eppendorf Brand Pipette/Tips
- MALDI Biotyper Target Worksheet
- Fresh Microorganism: overnight growth should be used for routine microorganism identification; slow-growing bacteria may need to cultivate for several days before testing. Do not use organisms that have been stored at 40C or lower, as this has a negative impact on quality of spectra and reproducibility; storing plates at room temperature for several days is acceptable. Different media types and growth temperatures have little effect on results. PREPARE CULTURES FOR MALDI USING FORMIC ACID EXTRACTION IN LAB.
- Bacterial Test Standard (BTS)
TARGET LOADING:
1. Prepare a MALDI Target Worksheet with appropriate sample identification, assigning the first two target spots for BTS (QC) and the rest for each isolate to be analyzed.
2. Inoculate 1 µL of each Isolate Formic Acid extract supernatant (avoid touching the pellet) onto the target spots indicated on the Target Worksheet.
3. Once all the isolate extracts are loaded.
4. One student will place 1μL of BTS on each of the 2 BTS spots.
5. Air Dry all spots at Room temperature.
6. Immediately after BTS spots (and all other spots) are dry, overlay all spots with 1 µL of HCCA (Matrix) (to avoid oxidation of the standard). Once the HCCA is uncapped work quickly to avoid evaporation!!!
7. Be sure the target plate is completely dry before placing the Target plate in the MALDI-TOF.
8. You are ready to analyze your isolates using the MALDI-TOF MS.
Finishing the project
This is your last wet lab! After you have recorded all your results on a lab Google Doc, you need to freeze your isolates. Once you have frozen stocks, please discard all cultures. You will receive 5 "clean-up points" if we do not find any evidence of your cultures or tests and all of your isolates are clearly labeled in the freezer rack. If we have to discard and clean-up later after you, you will not receive any of those 5 points. The date that clean up will be assessed will be announced by your instructor.
Analyze isolates for ability to digest cellulose, starch, and to solubolize phosphate
Cellulose medium: The red color of the medium provides increased contrast between the cellulose containing medium and the halo or clear zone that indicates that cellulase has diffused out of bacterial producers and degraded the cellulose in the immediate vicinity.
PVK medium: Look for a clear zone around the inoculum indicating the phosphate in the medium was solubilized and processed by enzymes secreted from the bacteria.
Starch Medium: Flood the plate with Grams Iodine. Let the plate sit for at least 10 minutes, longer is fine. The plate should turn dark blue as the iodine binds to the starch. Pour off the excess iodine (into paper towels in the biohazard bag on your bench) and wait until the remaining liquid is absorbed by the plate. Look for the presence of a clear halo around the inoculum site indicating the presence of amylase.
Finish Freezing freshly cultured isolate protocol
Please complete the Google doc prior to freezing your isolate. After you have recorded all your results, follow these instructions (also found in protocols) to freeze your isolates.
FREEZING “PURE” bacterial CULTURES FROM STOCKS
- microfuge tubes
- 1000µL Pipetman
- microfuge tube labels
- sterile 20% glycerol stock
- sterile toothpicks
- young culture (~24 hr) of student pure isolates grown on plates
- Google Doc or Excel Spreadsheet for Strain Database
Remember that your frozen stocks are potentially the one and only source you have for that bacterial species. Therefore be especially careful with your sterile technique when creating and working with your stocks, and make sure you are vigilant about keeping your strain list up-to-date and correct
Wear gloves to avoid contamination and work near flame (watch your hair and hands!)
Thoroughly wash your hands and decontaminate the bench before beginning
Make sure not to place tube cap on any surface (to keep it sterile)
Create a strain database file
- Give each isolate a strain name: your initials followed by a number
- Use the 209 lab Google Doc spreadsheet and fill in all the information listed below
- Strain name
- Bacterial species (if known)
- The source of the bacterial strain (person or environment)
- Any known genotype information or mutations
- The date the stock was made
- Information about where in your notebook you first discuss this strain
- The source of the bacterial strain (person or environment)
- Any known genotype information or mutations
- The date the stock was made
- Information about where in your notebook you first discuss this strain
Making the Cell Stock
1. Label the side of the cryo-tube with the cell strain name (and any additional info you want)
2. Add scotch tape over the label to ensure it doesn’t rub off
3. If the cryo-tube has a place to put a top label on, prepare that also
NOTE: The cryo-tubes typically do not fit into the standard eppendorf racks; a good alternative is to use the styrofoam base that 15 ml conicals come in—those openings are large enough for the cryo-tubes to rest securely in but not so deep you cannot remove and replace the cap easily
4. Screw open the tube but leave the cap sitting loosely on top
5. Add 800 ul of 20% glycerol in LB to the cryo-tube using P1000 pipette (be careful to not set cap down or let it touch any surface)
6. Scrape up cells from the plate with a flat toothpick—try not to gouge agar, just remove cells
7. Open cap and swirl toothpick in the media/glycerol to remove cells
8. Place 2 – 3 good-sized scrapings into the glycerol media
9. Pipette gently with P1000 to break up chunks of cells and disperse them
10. Mixture should be opaque—if not, add more cells
11. Place in -80 degree freezer in labeled freezer box
Be sure to label both the lid and the tube of a glycerol stock before you place the sample at -80°C. Frozen tubes are hard to write on and samples stored for long periods at -80°C can lose labels stuck to tube!
Tips and FAQ
The optimal concentration of long-term glycerol storage is unknown. Most labs store bacteria in 15-25% glycerol.
Try not to freeze/thaw your glycerol stock too many times. If you need to thaw frequently than make a new glycerol stock replacement from a freshly grown culture.
Assignment
Study for your Lab Practical
More information at Lab Practical tips
To Do:
Discard and clean up any remaining test materials and old cultures of your isolates. Note that there are 5 bonus points awarded for perfect clean-up. Your instructor will explain the criteria for obtaining these points.
Work on your final paper. Instructions at Final Paper
.
Links to Labs
Lab 1
Lab 2
Lab 3
Lab 4
Lab 5
Lab 6
Lab 7
Lab 8
Lab 9
Lab 10
Lab 11