BISC209/F13: Lab7: Difference between revisions
Tucker Crum (talk | contribs) |
Tucker Crum (talk | contribs) |
||
| (42 intermediate revisions by 2 users not shown) | |||
| Line 3: | Line 3: | ||
<div style="padding: 10px; width: 720px; border: 5px solid #483D8B;"> | <div style="padding: 10px; width: 720px; border: 5px solid #483D8B;"> | ||
=='''LAB 7: Diversity | =='''LAB 7: Structural Diversity in Cultured Isolates Con't & Testing Isolates for Positive/ Negative Interactions'''== | ||
<font size="+1"> | <font size="+1">Confirmation of Gram stain results by Selective/Differential Media:</font size="+1"><BR> | ||
Did each of your isolates grow on PEA or EMB? What does that result mean about the isolate's cell wall composition? Do your Gram stain findings and PEA and EMB growth patterns agree? If not, how might you explain unexpected findings?<BR><BR> | Did each of your isolates grow on PEA or EMB? What does that result mean about the isolate's cell wall composition? Do your Gram stain findings and PEA and EMB growth patterns agree? If not, how might you explain unexpected findings? If you have time today, repeat Gram stains on any isolates whose PEA or EMB growth did not match your Gram stain finding<BR><BR> | ||
=='''MOTILITY '''== | |||
Every isolate should be inoculated into a soft agar deep of nutrient agar with a low agar content. "Soft" agar contains much less solidifying agent (agar) than most solid media (0.35% rather than the usual 1.5%). The growth pattern in this semi-solid NA medium gives information about motility. <BR> | |||
A full description of these tests can be found in the protocols section: [[BISC209/F13: Motility | Motility Tests]]. <BR> | |||
If either of your motility test are positive when we analyze the results, you could try to confirm motility with a hanging drop technique or by trying a flagella stain. <BR><BR> | |||
'''PROTOCOL:'''<BR> | |||
Inoculating a soft agar deep involves a technique you have not yet practiced. You will use an inoculating needle: the wire extending from the handle of the needle will not have a loop on the end.<BR> | |||
1) You will need a tube of soft nutrient agar medium for each of your isolates. Each class needs to add 2 extra tubes of soft agar medium to inoculate one with ''E. coli'', an organism that is motile, which will serve as a positive motility control and the other with ''Staphylococcus epidermidis'', an organism that is non-motile, to serve as our negative motility control.<BR><BR> | |||
2). Flame sterilize an inoculating needle, cool it for a few seconds, and pick up a barely visible amount of colony growth on the tip of the needle. <BR><BR> | |||
3) Stab the needle with the inoculum deeply into the center of the soft agar tube, stopping just before the bottom of the tube or, if you are running out of needle, stab it until the you are almost to the end of the needle.<BR><BR> | |||
4) Withdraw the needle through the same inoculation channel. (This procedure is also known as "making a soft agar deep".) <BR><BR> | |||
5) Inoculate each of your other isolates using the same technique.<BR><BR> | |||
6) Make sure the class has inoculated a positive control tube of ''E. coli''and a negative control tube of ''S. epidermidis''.<BR><BR> | |||
7) Incubate all tubes for 24-72 hours. The tubes should be incubated at RT. <BR><BR> | |||
8) After sufficient growth is observed in the tube, look for a color change from red to yellow. Use of mannitol as a carbon source results in a pH change that we can see using phenol red indicator (change from red to yellow).<BR> | |||
9) Check for motility by looking for diffuse growth radiating from the stab line of inoculation. Compare the motility of each of your isolates to that of the ''E. coli'' positive control and the Staph negative control. ''E. coli'' are a motile bacteria and all ''Staphyloccus'' species are non-motile. <BR><BR> | |||
'''Control Organisms:'''<BR> | |||
{| border="1" | |||
|+ | |||
! Organism !! ATCC !! Motility | |||
|- | |||
! ''Escherichia coli'' | |||
| 25922 | |||
| + | |||
|- | |||
! ''Klebsiella pneumoniae'' | |||
| 13883 | |||
| - | |||
|- | |||
! ''Proteus mirabilis'' | |||
| 25933 | |||
| + | |||
|- | |||
! ''Acinobacter anitrartum'' | |||
| 17924 | |||
| - | |||
|- | |||
|} | |||
<br> | |||
==McFarland Turbidity Standards== | |||
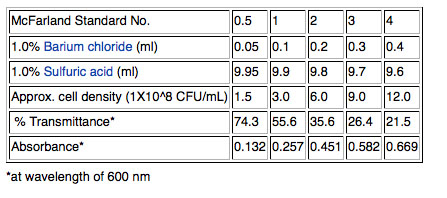
1) Although cell density can be measured more accurately using a spectrophotometer to measure optical density (OD) at 600nm, we will use a quicker method that will work well enough for our purposes. We will use a McFarland 0.5 standard; the 0.5 refers to the approximate concentration of organisms in solutions which is 1.5X10<sup>8</sup> cfu/mL for the 0.5 standard. Other common standards are shown in the table below. <BR> | |||
2) You will use the fresh cultures of each of your isolates that you prepared prior to lab for both the antibiotic and interactions protocols. We want to apply relatively the same number of organisms in each procedure so we will use McFarland Standard to "correct" turbidity. <BR> | |||
3. Label with your isolate code a 13 X 100 ml capped tube pre-aliquoted with 2 mL of sterile water for each isolate. <BR> | |||
4. Use your flamed and cooled loop to pick up a visible amount of growth from your freshly grown cultures. <BR> | |||
5. Using your best aseptic technique transfer the bacteria to the water and dislodge the bacteria by gently mixing your loop in the water.<BR> | |||
6. Vortex gently. <BR> | |||
7.Compare the turbidity in your tube of diluted isolate to the 0.5 McFarland Standard provided.<BR> | |||
8. If they are unequal in turbidity, aseptically add more culture or more sterile water until the cloudiness (turbidity) of the two tubes appears visually similar in cloudiness. Do not obsess over the match, just be close. <BR> | |||
9. Repeat for each of your isolates.<BR> | |||
10. If you have filamentous bacteria, those hard powdery white topped colonies you may have a difficult time getting the turbidity to match, in this case, remove 1 ml of the water + backeria mix to an empty sterile 13 X 100 capped tube and add more bacteria to this smaller volume. Vortex and use this heavier inoculum for setting up your tests today. Place the original tube in a rack on the discard bench so that you will not mix it up with the heavier inoculum.<BR> | |||
11. The ending volume is irrelevant. Vortex to mix. <BR> | |||
12. You will use these tubes as a source of organism for both the antibiotics and interactions assays today. <BR> | |||
<BR> | |||
[[Image:mcfarlandtable.jpg]]<BR> | |||
==Test for Antibiotic Production== | |||
Many microbes secrete antimicrobial compounds to help them compete with other microorganisms for habitat. Some of the bacteria that are common antibiotic producers are the Actinomycetes (including ''Streptomycetes'' species), many of the ''Bacillus'' species, and the fruiting myxobacteria, to name just a few among many, many antibiotic producing bacteria. You can also test for the opposite: the sensitivity of your soil organisms (or known stock bacteria) to manufactured or secreted antibiotics.<BR> <BR> | |||
(This testing will take 3 weeks.) <BR> | |||
''' Week 1:''' <BR> | |||
Identify how many potential antibiotic producers you might have. Definitely test any isolates that are likely to be ''Actinomycetes'', ''Myxobacteria'', or ''Bacillus''. It is wise to test all of your isolates since the soil is the main source of microbes that supply the world's antibiotics. It's possible that you might discover the next great antimicrobial drug and get very rich by selling the patent for your discovery to a drug company. Remember that the discovery of penicillin was completely accidental. <BR><BR> | |||
1. Using aseptic technique dip a sterile swab into the concentration adjusted culture tube you made to match the turbidity of the McFarland 0.5 standard and make an inoculation (as shown below) down the middle of a plate of nutrient agar. <BR> | |||
2. Make additional plates exactly like the first for each isolate to be tested. Label them carefully and incubate the plates for ~1 week at RT.<BR><BR> | |||
[[Image:strep1a.jpg]]<BR> | |||
==Antagonistic and Mutualistic Interactions Analysis== | ==Antagonistic and Mutualistic Interactions Analysis== | ||
The microbial community living in soil is a complex one with many different microorganisms. As is true of any environment, these microbes interact with each other - both functionally and physically. Do selected bacteria from your community help each other or harm each other while trying to find a niche in your soil community? Today, you will try to answer that question by testing your cultured isolates for examples of mutualism or antagonism (co-operation or competition)by culturing them in controlled communities. Some of these bacteria may prevent the growth of others through the production of chemical inhibitors; others might promote the growth of their neighbors by producing metabolites that are needed. We are going to look for both positive and negative interactions.<br> | The microbial community living in soil is a complex one with many different microorganisms. As is true of any environment, these microbes interact with each other - both functionally and physically. Do selected bacteria from your community help each other or harm each other while trying to find a niche in your soil community? Today, you will try to answer that question by testing your cultured isolates for examples of mutualism or antagonism (co-operation or competition) by culturing them in controlled communities. Some of these bacteria may prevent the growth of others through the production of chemical inhibitors; others might promote the growth of their neighbors by producing metabolites that are needed. We are going to look for both positive and negative interactions.<br> | ||
'''PREPARING THE ISOLATES''':<BR> | '''PREPARING THE ISOLATES''':<BR> | ||
<font size="+1">Interaction Assay Set Up</font size="+1"> | <font size="+1">Interaction Assay Set Up</font size="+1"> | ||
| Line 33: | Line 106: | ||
[[Image:Interactions_slide2.jpg]] <BR> | [[Image:Interactions_slide2.jpg]] <BR> | ||
<BR> | <BR> | ||
You will use 64 of the wells on a 96 well plate for this assay. Each assay will use 8 | You will use 64 of the wells on a 96 well plate for this assay. to do this each team member will contribute two unique organisms. Each assay will use 8 isolates to test for interactions. Use the Excel template provided [[Media:template.xls]] to record the identifying codes of the organisms that will be inoculated into each well as described and illustrated below.<BR> | ||
| Line 40: | Line 113: | ||
[[Image:Interactions_slide3.jpg]]<BR> | [[Image:Interactions_slide3.jpg]]<BR> | ||
1. You will inoculate 50 µl of each of the 8 unique isolates to be tested into the illustrated row of wells. Use the concentration adjusted culture tube you made to match the turbidity of the McFarland 0.5 standard since you are trying to control for similar numbers of organisms in your inoculum. (A1 is Isolate #1(use your code id), A2 is Isolate #2 etc through A8) | |||
[[Image:Interactions_slide4.jpg]] <BR> | [[Image:Interactions_slide4.jpg]] <BR> | ||
Beginning with Isolate #2, inoculate a second 50 μl of each of your isolates into the column wells B1, C1, etc. (indicated by the green color). | 2. Beginning with Isolate #2 (''do not add a second 50 µL to well A1''), inoculate a second 50 μl of each of your isolates into the column wells B1, C1, etc. (indicated by the green color). | ||
<BR> | <BR> | ||
Add 100 μL of nutrient broth to each of the wells containing your isolates (row wells A1-A8 and column wells B1-H1) | 3. Add 100 μL of nutrient broth to each of the wells containing your isolates (row wells A1-A8 and column wells B1-H1) | ||
<BR> | <BR> | ||
Gently move the 96 well plate in a circular motion to mix.<BR> | 4. Gently move the 96 well plate in a circular motion to mix.<BR> | ||
| Line 56: | Line 129: | ||
[[Image:Interactions_slide5.jpg]] <BR> | [[Image:Interactions_slide5.jpg]] <BR> | ||
<BR> | <BR> | ||
5. Use an 8 channel multichannel pipet with the first tip removed (you are not yet transferring from well A1) to transfer 10 μl of the contents of the 7 wells in the top row: A2 (containing isolate #2 etc) through A8 to the corresponding empty wells in each row as indicated by the yellow arrows. Change tips between each transfer from row 1 to row 2, then row 1 to row 3, etc. until each row indicated by the yellow arrows has been inoculated with 10 µL from the corresponding well in row 1. <BR> | |||
<BR> | <BR> | ||
If you are using the multichannel pipette, be sure that you work slowly and check that each pipette tip is evenly filled. You may need to tighten the tips by hand, if so be sure to only touch the part of the tip that sits on the multichannel pipette, you wouldn't want to contaminate your wells with human organsisms!<BR><BR> | |||
[[Image:Interactions_slide6.jpg]] <BR> | [[Image:Interactions_slide6.jpg]] <BR> | ||
<BR> | <BR> | ||
6. Using all 8 channels of a multichannel pipet, transfer 10 μl of the contents of column 1 (all 8 wells) to each of the corresponding wells in the other columns. Again, change tips between each transfer: A1 through H1, to A2 to H2 then A1 through H1 to A3 to H3 etc. until each of the columns indicated by the red arrows has been inoculated with 10 µL from the corresponding well in column 1. <BR> | |||
7. Mix the contents of the well by moving the plate in gentle circles.<BR> | |||
<BR> | <BR> | ||
NEXT, we will inoculate a square (NUNC) tray | 8. NEXT, we will inoculate a labeled square (NUNC) tray containing nutrient agar medium with about 5 μl of the contents of the wells we just prepared. For this step we will use either a tool called a "frogger" or a multichannel micropipette. If using the frogger, dip the frogger tips into 96 well plate to attract a drop of inoculum onto the end of each steel tip. Be sure the frogger is actually sitting in the liquid of all 64 wells, you should make sure that the tips rest on the bottom of the plate. Transfer the Frogger to the surface of the sterile NA in the square NUNC plate. Place the Frogger gently on the agar and release the handle. As long as you don't push down when the frogger is touching the medium, you will not break the surface but you will get a transfer to the NUCN plate of the cultures in each of the 64 wells. Just to be sure that all were transferred, look carefully at the NUNC plate after you have cleaned the frogger (see below). If there is a "spot" missing (you should be able to see residue from each culture on the surface of the NUNC plate), use your P20 set to 5 microliters and put 5 microliters of the missing culture in the appropriate spot. | ||
Be sure to disinfect the frogger by dipping it into a series of disinfectant and rinse solutions that you will find at the '''cleaning station''' prepared for you . <BR><BR> | |||
9. If the frogger is not available, use an 8 channel multichannel pipet set to 5µl and remove 5μL of culture from each well of your culture dish and deposit all of it onto an area of the NA square agar NUNC plate that is in the same location as in the 96 well culture dish. Again, be sure the tips are on tightly before loading the pipet. Repeat this procedure, with new tips, for each ROW of 8 wells until you have completed depositing the full array in the same orientation as the 48 wells.<br><BR> | |||
10. Wait for your inoculated spots to dry, seal or cover the NUNC square tray, and incubate at Room Temp. The 96 well plate was used only to mix the cultures so you can discard this in your autoclave bag. <br> | |||
==Pictionary® Tournament== | |||
Your instructor will explain the rules of the Pictionary® game we will play using the knowledge you now have about tools and techniques of microbiologists. | |||
==CLEAN UP== | ==CLEAN UP== | ||
| Line 99: | Line 179: | ||
12. Wash your hands. | 12. Wash your hands. | ||
==Assignment== | ==Assignment== | ||
'''Graded Assignment:''' <BR> | '''Graded Assignment:''' <BR> | ||
There is no graded assignment for next lab:)<BR> | |||
''' | '''TO DO''':<BR> | ||
You will need a VERY fresh culture of your bacterial isolates in the next lab for our attempt to identify them using our MALDI-TOF BioTyper. Come into the lab 18-24 hours before lab to set up a fresh sub-culture on a new NA plate for each of your isolates. <BR> | |||
<div class=noprint> | <div class=noprint> | ||
Latest revision as of 10:16, 8 November 2013
LAB 7: Structural Diversity in Cultured Isolates Con't & Testing Isolates for Positive/ Negative Interactions
Confirmation of Gram stain results by Selective/Differential Media:
Did each of your isolates grow on PEA or EMB? What does that result mean about the isolate's cell wall composition? Do your Gram stain findings and PEA and EMB growth patterns agree? If not, how might you explain unexpected findings? If you have time today, repeat Gram stains on any isolates whose PEA or EMB growth did not match your Gram stain finding
MOTILITY
Every isolate should be inoculated into a soft agar deep of nutrient agar with a low agar content. "Soft" agar contains much less solidifying agent (agar) than most solid media (0.35% rather than the usual 1.5%). The growth pattern in this semi-solid NA medium gives information about motility.
A full description of these tests can be found in the protocols section: Motility Tests.
If either of your motility test are positive when we analyze the results, you could try to confirm motility with a hanging drop technique or by trying a flagella stain.
PROTOCOL:
Inoculating a soft agar deep involves a technique you have not yet practiced. You will use an inoculating needle: the wire extending from the handle of the needle will not have a loop on the end.
1) You will need a tube of soft nutrient agar medium for each of your isolates. Each class needs to add 2 extra tubes of soft agar medium to inoculate one with E. coli, an organism that is motile, which will serve as a positive motility control and the other with Staphylococcus epidermidis, an organism that is non-motile, to serve as our negative motility control.
2). Flame sterilize an inoculating needle, cool it for a few seconds, and pick up a barely visible amount of colony growth on the tip of the needle.
3) Stab the needle with the inoculum deeply into the center of the soft agar tube, stopping just before the bottom of the tube or, if you are running out of needle, stab it until the you are almost to the end of the needle.
4) Withdraw the needle through the same inoculation channel. (This procedure is also known as "making a soft agar deep".)
5) Inoculate each of your other isolates using the same technique.
6) Make sure the class has inoculated a positive control tube of E. coliand a negative control tube of S. epidermidis.
7) Incubate all tubes for 24-72 hours. The tubes should be incubated at RT.
8) After sufficient growth is observed in the tube, look for a color change from red to yellow. Use of mannitol as a carbon source results in a pH change that we can see using phenol red indicator (change from red to yellow).
9) Check for motility by looking for diffuse growth radiating from the stab line of inoculation. Compare the motility of each of your isolates to that of the E. coli positive control and the Staph negative control. E. coli are a motile bacteria and all Staphyloccus species are non-motile.
Control Organisms:
| Organism | ATCC | Motility |
|---|---|---|
| Escherichia coli | 25922 | + |
| Klebsiella pneumoniae | 13883 | - |
| Proteus mirabilis | 25933 | + |
| Acinobacter anitrartum | 17924 | - |
McFarland Turbidity Standards
1) Although cell density can be measured more accurately using a spectrophotometer to measure optical density (OD) at 600nm, we will use a quicker method that will work well enough for our purposes. We will use a McFarland 0.5 standard; the 0.5 refers to the approximate concentration of organisms in solutions which is 1.5X108 cfu/mL for the 0.5 standard. Other common standards are shown in the table below.
2) You will use the fresh cultures of each of your isolates that you prepared prior to lab for both the antibiotic and interactions protocols. We want to apply relatively the same number of organisms in each procedure so we will use McFarland Standard to "correct" turbidity.
3. Label with your isolate code a 13 X 100 ml capped tube pre-aliquoted with 2 mL of sterile water for each isolate.
4. Use your flamed and cooled loop to pick up a visible amount of growth from your freshly grown cultures.
5. Using your best aseptic technique transfer the bacteria to the water and dislodge the bacteria by gently mixing your loop in the water.
6. Vortex gently.
7.Compare the turbidity in your tube of diluted isolate to the 0.5 McFarland Standard provided.
8. If they are unequal in turbidity, aseptically add more culture or more sterile water until the cloudiness (turbidity) of the two tubes appears visually similar in cloudiness. Do not obsess over the match, just be close.
9. Repeat for each of your isolates.
10. If you have filamentous bacteria, those hard powdery white topped colonies you may have a difficult time getting the turbidity to match, in this case, remove 1 ml of the water + backeria mix to an empty sterile 13 X 100 capped tube and add more bacteria to this smaller volume. Vortex and use this heavier inoculum for setting up your tests today. Place the original tube in a rack on the discard bench so that you will not mix it up with the heavier inoculum.
11. The ending volume is irrelevant. Vortex to mix.
12. You will use these tubes as a source of organism for both the antibiotics and interactions assays today.
Test for Antibiotic Production
Many microbes secrete antimicrobial compounds to help them compete with other microorganisms for habitat. Some of the bacteria that are common antibiotic producers are the Actinomycetes (including Streptomycetes species), many of the Bacillus species, and the fruiting myxobacteria, to name just a few among many, many antibiotic producing bacteria. You can also test for the opposite: the sensitivity of your soil organisms (or known stock bacteria) to manufactured or secreted antibiotics.
(This testing will take 3 weeks.)
Week 1:
Identify how many potential antibiotic producers you might have. Definitely test any isolates that are likely to be Actinomycetes, Myxobacteria, or Bacillus. It is wise to test all of your isolates since the soil is the main source of microbes that supply the world's antibiotics. It's possible that you might discover the next great antimicrobial drug and get very rich by selling the patent for your discovery to a drug company. Remember that the discovery of penicillin was completely accidental.
1. Using aseptic technique dip a sterile swab into the concentration adjusted culture tube you made to match the turbidity of the McFarland 0.5 standard and make an inoculation (as shown below) down the middle of a plate of nutrient agar.
2. Make additional plates exactly like the first for each isolate to be tested. Label them carefully and incubate the plates for ~1 week at RT.
Antagonistic and Mutualistic Interactions Analysis
The microbial community living in soil is a complex one with many different microorganisms. As is true of any environment, these microbes interact with each other - both functionally and physically. Do selected bacteria from your community help each other or harm each other while trying to find a niche in your soil community? Today, you will try to answer that question by testing your cultured isolates for examples of mutualism or antagonism (co-operation or competition) by culturing them in controlled communities. Some of these bacteria may prevent the growth of others through the production of chemical inhibitors; others might promote the growth of their neighbors by producing metabolites that are needed. We are going to look for both positive and negative interactions.
PREPARING THE ISOLATES:
Interaction Assay Set Up
You will use 64 of the wells on a 96 well plate for this assay. to do this each team member will contribute two unique organisms. Each assay will use 8 isolates to test for interactions. Use the Excel template provided Media:template.xls to record the identifying codes of the organisms that will be inoculated into each well as described and illustrated below.
FOLLOW THE TEMPLATE CAREFULLY!!!!!! It is easy to get this inoculation messed up, but don't!

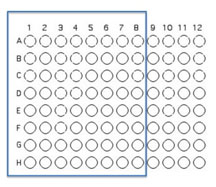
1. You will inoculate 50 µl of each of the 8 unique isolates to be tested into the illustrated row of wells. Use the concentration adjusted culture tube you made to match the turbidity of the McFarland 0.5 standard since you are trying to control for similar numbers of organisms in your inoculum. (A1 is Isolate #1(use your code id), A2 is Isolate #2 etc through A8)
2. Beginning with Isolate #2 (do not add a second 50 µL to well A1), inoculate a second 50 μl of each of your isolates into the column wells B1, C1, etc. (indicated by the green color).
3. Add 100 μL of nutrient broth to each of the wells containing your isolates (row wells A1-A8 and column wells B1-H1)
4. Gently move the 96 well plate in a circular motion to mix.
5. Use an 8 channel multichannel pipet with the first tip removed (you are not yet transferring from well A1) to transfer 10 μl of the contents of the 7 wells in the top row: A2 (containing isolate #2 etc) through A8 to the corresponding empty wells in each row as indicated by the yellow arrows. Change tips between each transfer from row 1 to row 2, then row 1 to row 3, etc. until each row indicated by the yellow arrows has been inoculated with 10 µL from the corresponding well in row 1.
If you are using the multichannel pipette, be sure that you work slowly and check that each pipette tip is evenly filled. You may need to tighten the tips by hand, if so be sure to only touch the part of the tip that sits on the multichannel pipette, you wouldn't want to contaminate your wells with human organsisms!
6. Using all 8 channels of a multichannel pipet, transfer 10 μl of the contents of column 1 (all 8 wells) to each of the corresponding wells in the other columns. Again, change tips between each transfer: A1 through H1, to A2 to H2 then A1 through H1 to A3 to H3 etc. until each of the columns indicated by the red arrows has been inoculated with 10 µL from the corresponding well in column 1.
7. Mix the contents of the well by moving the plate in gentle circles.
8. NEXT, we will inoculate a labeled square (NUNC) tray containing nutrient agar medium with about 5 μl of the contents of the wells we just prepared. For this step we will use either a tool called a "frogger" or a multichannel micropipette. If using the frogger, dip the frogger tips into 96 well plate to attract a drop of inoculum onto the end of each steel tip. Be sure the frogger is actually sitting in the liquid of all 64 wells, you should make sure that the tips rest on the bottom of the plate. Transfer the Frogger to the surface of the sterile NA in the square NUNC plate. Place the Frogger gently on the agar and release the handle. As long as you don't push down when the frogger is touching the medium, you will not break the surface but you will get a transfer to the NUCN plate of the cultures in each of the 64 wells. Just to be sure that all were transferred, look carefully at the NUNC plate after you have cleaned the frogger (see below). If there is a "spot" missing (you should be able to see residue from each culture on the surface of the NUNC plate), use your P20 set to 5 microliters and put 5 microliters of the missing culture in the appropriate spot.
Be sure to disinfect the frogger by dipping it into a series of disinfectant and rinse solutions that you will find at the cleaning station prepared for you .
9. If the frogger is not available, use an 8 channel multichannel pipet set to 5µl and remove 5μL of culture from each well of your culture dish and deposit all of it onto an area of the NA square agar NUNC plate that is in the same location as in the 96 well culture dish. Again, be sure the tips are on tightly before loading the pipet. Repeat this procedure, with new tips, for each ROW of 8 wells until you have completed depositing the full array in the same orientation as the 48 wells.
10. Wait for your inoculated spots to dry, seal or cover the NUNC square tray, and incubate at Room Temp. The 96 well plate was used only to mix the cultures so you can discard this in your autoclave bag.
Pictionary® Tournament
Your instructor will explain the rules of the Pictionary® game we will play using the knowledge you now have about tools and techniques of microbiologists.
CLEAN UP
1. All culture plates that you are finished with should be discarded in the big orange autoclave bag near the sink next to the instructor table. Ask your instructor whether or not to save stock cultures and plates with organisms that are provided.
2. Culture plates, stocks, etc. that you are not finished with should be labeled on a piece of your your team color tape. Place the labeled cultures in your lab section's designated area in the incubator, the walk-in cold room, or at room temp. in a labeled rack. If you have a stack of plates, wrap a piece of your team color tape around the whole stack.
3. Remove tape from all liquid cultures in glass tubes. Then place the glass tubes with caps in racks by the sink near the instructor's table. Do not discard the contents of the tubes.
4. Glass slides or disposable glass tubes can be discarded in the glass disposal box.
5. Make sure all contaminated, plastic, disposable, serologic pipets and used contaminated micropipet tips are in the small orange autoclave bag sitting in the plastic container on your bench.
6. If you used the microscope, clean the lenses of the microscope with lens paper, being very careful NOT to get oil residue on any of the objectives other than the oil immersion 100x objective. Move the lowest power objective into the locked viewing position, turn off the light source, wind the power cord, and cover the microscope with its dust cover before replacing the microscope in the cabinet.
7. If you used it, rinse your staining tray and leave it upside down on paper towels next to your sink.
8. Turn off the gas and remove the tube from the nozzle. Place your bunsen burner and tube in your large drawer.
9. Place all your equipment (loop, striker, sharpie, etc) including your microfuge rack, your micropipets and your micropipet tips in your small or large drawer.
10. Move your notebook and lab manual so that you can disinfect your bench thoroughly.
11. Take off your lab coat and store it in the blue cabinet with your microscope.
12. Wash your hands.
Assignment
Graded Assignment:
There is no graded assignment for next lab:)
TO DO:
You will need a VERY fresh culture of your bacterial isolates in the next lab for our attempt to identify them using our MALDI-TOF BioTyper. Come into the lab 18-24 hours before lab to set up a fresh sub-culture on a new NA plate for each of your isolates.