BISC110/F12: Lab 8: Difference between revisions
Tucker Crum (talk | contribs) |
No edit summary |
||
| (4 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{Template:BISC110/ | {{Template:BISC110/S13}} | ||
<div style="padding: 10px; width: 725px; border: 5px solid # | <div style="padding: 10px; width: 725px; border: border: 5px solid #00cc66;"> | ||
=='''Part 1: Spectrophotometry and Creating a Standard Curve'''== | =='''Part 1: Spectrophotometry and Creating a Standard Curve'''== | ||
Your lab today is divided into two distinct parts. In the first portion of the lab, you will measure the absorption of a blue pigmented solution of the chemical compound, 2,6-dichlorophenol indophenol (DCPIP) at a range of wavelengths to determine its wavelength of maximum absorbance. You will measure absorbance at this maximum wavelength of several dilutions of this solution and use these data to generate a standard curve by plotting absorbance against concentration. This curve will allow you to calculate the concentration of an unknown. In the later portion of the lab, you will participate in a writing workshop for the data collected in labs 6 and 7 (a completion of the Taster Series 2).<BR> | Your lab today is divided into two distinct parts. In the first portion of the lab, you will measure the absorption of a blue pigmented solution of the chemical compound, 2,6-dichlorophenol indophenol (DCPIP) at a range of wavelengths to determine its wavelength of maximum absorbance. You will measure absorbance at this maximum wavelength of several dilutions of this solution and use these data to generate a standard curve by plotting absorbance against concentration. This curve will allow you to calculate the concentration of an unknown. In the later portion of the lab, you will participate in a writing workshop for the data collected in labs 6 and 7 (a completion of the Taster Series 2).<BR> | ||
| Line 50: | Line 50: | ||
#In the main menu, choose wavelength scan | #In the main menu, choose wavelength scan | ||
#Wavelength scan (350nm - 700nm). (Make sure that the y axis is showing Abs (absorbance) rather than % transmittance.) | #Wavelength scan (350nm - 700nm). (Make sure that the y axis is showing Abs (absorbance) rather than % transmittance.) | ||
#Put in cuvette with water as your instrument blank. Make sure that the clear side of your cuvette is directed so that the clear side is facing the | #Put in cuvette with water as your instrument blank. Make sure that the clear side of your cuvette is directed so that the clear side is facing the light path (back/front) for this spec and not right/left as you would if using the DU530 specs. | ||
#Press Blank | #Press Blank | ||
#When the blanking is complete put in cuvette with sample. Make sure that the clear side of your cuvette is directed facing the light path.<br> | #When the blanking is complete put in cuvette with sample. Make sure that the clear side of your cuvette is directed facing the light path.<br> | ||
| Line 80: | Line 80: | ||
<font size="+1">'''C. Determination of Absorbance'''</font size="+1"><br> | <font size="+1">'''C. Determination of Absorbance'''</font size="+1"><br> | ||
Use of the Spectronic20+ Spectrophotometer <BR> | Use of the Spectronic20+ Spectrophotometer <BR> | ||
You will find a different spectrophotometer at the end of your lab bench. This Spec20+ is used to measure absorbance at a set wavelength. '''NOTE that the Spec 20 instruments | You will find a different spectrophotometer at the end of your lab bench. This Spec20+ is used to measure absorbance at a set wavelength. '''NOTE that the Spec 20 instruments does NOT use square cuvettes!!!''' This instrument uses the round bottom disposable 13mm glass test tubes that you used when you made your dilutions. | ||
An animation of how to use a Spec 20+ can be found at: [http://www.wellesley.edu/Biology/Concepts/Html/analogspec20instructions.html | Spec20 Instructions]<br><br> | An animation of how to use a Spec 20+ can be found at: [http://www.wellesley.edu/Biology/Concepts/Html/analogspec20instructions.html | Spec20 Instructions]<br><br> | ||
| Line 145: | Line 145: | ||
=='''Assignment'''== | =='''Assignment'''== | ||
'''Turn in at the beginning of Lab 9'''<br> | '''Turn in at the beginning of Lab 9'''<br> | ||
1. Your completed TASTER paper is due at the beginning of Lab 9. Please write a partial scientific paper (Title, Abstract, Results, Discussion, and References). Your instructor will provide more detailed instructions during your writing workshop. <br> | 1. (35 points) Your completed TASTER paper is due at the beginning of Lab 9. Please write a partial scientific paper (Title, Abstract, Results, Discussion, and References). Your instructor will provide more detailed instructions during your writing workshop. This assignment must be done individually. <br> | ||
2. In your lab notebook, prepare a flow chart for the Hill Reaction (Lab 9).<br> | 2. In your lab notebook, prepare a flow chart for the Hill Reaction (Lab 9).<br> | ||
3. Review the mechanisms of electron transport and proton flow in photosynthesis in your textbook.<br> | 3. Review the mechanisms of electron transport and proton flow in photosynthesis in your textbook.<br> | ||
Latest revision as of 12:27, 10 January 2013
Part 1: Spectrophotometry and Creating a Standard Curve
Your lab today is divided into two distinct parts. In the first portion of the lab, you will measure the absorption of a blue pigmented solution of the chemical compound, 2,6-dichlorophenol indophenol (DCPIP) at a range of wavelengths to determine its wavelength of maximum absorbance. You will measure absorbance at this maximum wavelength of several dilutions of this solution and use these data to generate a standard curve by plotting absorbance against concentration. This curve will allow you to calculate the concentration of an unknown. In the later portion of the lab, you will participate in a writing workshop for the data collected in labs 6 and 7 (a completion of the Taster Series 2).
Introduction to Series 3--Spectrophotometry
SPECTROPHOTOMETRY
Light is a form of electromagnetic radiation. It travels through space in rhythmic waves, and the distance between the crests of the waves is called the wavelength. Wavelengths of electromagnetic radiation range from less than a nanometer to greater than a kilometer, and this wavelength is inversely proportional to the amount of energy present. The entire range of electromagnetic radiation is called the electromagnetic spectrum, which is diagrammed in Figure 3.
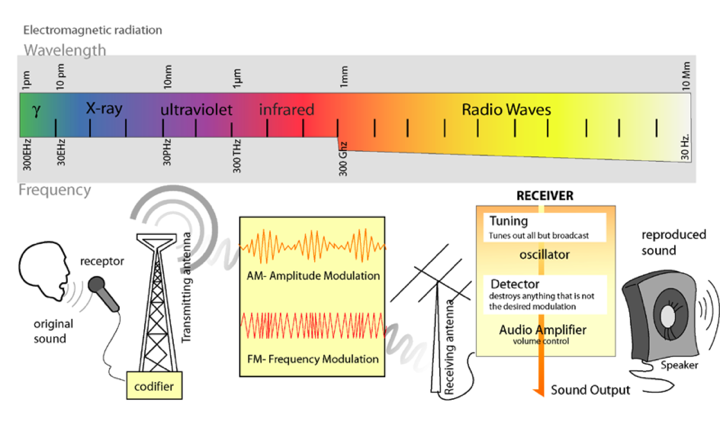
Figure 3. The Electromagnetic Spectrum. Image from: http://commons.wikimedia.org/wiki/File:Radio_transmition_diagram_en.png
The radiation in the visible portion of the spectrum is detected as colors by the human eye. Color is determined by the wavelengths of light that are reflected by molecules in the substance, while the other wavelengths are absorbed. Therefore, an object that appears red absorbs the blue-green wavelengths of light and reflects the red wavelengths.
Our perception of color is qualitative, but there are instruments that quantify the amount of light absorbed at a particular wavelength by a solution. These instruments are called spectrophotometers. As shown in Figure 4, a simple spectrophotometer contains a light source that is focused on a prism or diffraction grating, which splits this light into individual energy bands. These bands are further focused through a narrow slit, which can be moved across the spectrum to select a specific wavelength. This monochromatic (one wavelength) beam of light is passed through a cuvette containing the sample to be measured. As the light passes through the sample, some of it is absorbed by the molecules in the solution. The remainder of the light is transmitted through the sample, and impacts a photodetector that changes light energy into an electrical current. The magnitude of this current is proportional to the light intensity. The detector signal is amplified by a photomultiplier and is fed into the display meter as a number (digital) or a stylus deflection (analog), which indicates the amount of light absorbed by the molecules in the solution. Most spectrophotometers can also indicate the amount of light that is transmitted through the solution.

Figure 4. Diagram of the Light Paths in a Spectrophotometer. Image from:
http://commons.wikimedia.org/wiki/File:Spetrophotometer-en.svg
The Beer-Lambert Law
The amount of light that passes through a solution in a spectrophotometer is called transmittance (T), and can be defined mathematically as, T = I/Io, where I is the intensity of the light transmitted through the sample, and Io is the intensity of the incident light on the sample.
The absorbance (A) is the negative log of the transmittance, A = –log T. Absorbance is directly proportional to the concentration of the absorbing molecules in the solution and the distance the light travels through the solution. Therefore, absorbance can be used as a quantitative measure of the concentration of a solution. These concepts are expressed mathematically in the Beer-Lambert Law:

The molar extinction coefficient is a constant that is dependent upon the chemical nature of the absorbing material and the wavelength employed for the absorbance measurement. The path length is determined by the width or diameter of the cuvette used to contain the sample. Absorbance is unitless.
Quantitative analysis of many biological materials is based on: a) the material itself exhibiting light absorbance at a particular wavelength, or b) the biological material reacting with a chemical reagent to form a colored product in such a way that the absorbance of the product is a quantitative measure of the concentration of the biological material. Because these materials must be dissolved in a solvent before an absorbance reading can be obtained, absorption by the solvent is a potential source of error. To insure that the absorbance reading will reflect only the absorbance of the molecules to be quantified, spectrophotometers are initially set to zero absorbance with the solvent that was used to dissolve or dilute the solutions. The tube containing the solvent is called the blank.
Making and Using a Standard Curve
A.Determination of Peak Absorbance from an Absorption Spectrum
- In pairs, obtain two 13mm glass test tubes from the stock area in the front of the lab.
- Label one test tube "DCPIP" and the other "BLANK".
- With a 10.0 mL serological pipette add 6.0 mL distilled water to the "BLANK" tube and add 8.0 ml of the blue solution to the tube marked "DCPIP".
- Obtain two disposable plastic cuvettes and fill one with water and the other with the sample so that each of them is about 3/4 full. The exact amount is not important. It is not necessary to label these cuvettes; you will be able to tell the difference by color of the contents.
- Use either the BeckmanDU530 or 720 spectrophotometer to run an absorbance spectrum. Take your two cuvettes to one of the instruments.
If you are using the DU530 follow these instructions:
- From the Main Menu, press the soft key under “USER PROGRAM.” Select Program #5 (400-700nm, absorption spectra)
- Place a plastic cuvette filled at least 2/3 full with water into the cuvette holder oriented so that the clear sides are facing the light path , then press the soft key under Baseline to initiate the baseline scan of the blanking material. It will take several minutes to complete the baseline scan.
- When the baseline scan is completed the screen will read Ready to Scan.
- Remove the cuvette containing the water blank and insert the cuvette containing the sample. Make sure the clear side of the cuvette faces the light path (left and right)
- Press the soft key under Start Scan to initiate the wavelength scan, which will be displayed on the screen as it is being generated. After the spectrum is complete the peaks and valleys will be rescaled automatically as the instrument adjusts the range of % absorbance on the y-axis.
- To find out the peak absorbance wavelength, press the cursor soft key. Use the left and right pointing arrows on the bottom of the screeen to move the cursor to find your peak. The absorbance and the wavelength at in nanometers is displayed below the x axis of the graph on the screen. The best wavelength for measuring absorbance of DCPIP is seen as the peak but note that there is a range of wavelengths at which DCPIP can be measured. Record the peak wavelength in nm and the absorbance of undiluted DCPIP at this wavelength in your lab notebook.
- To print your absorbance spectrum, press the PRINT key on the keypad, followed by the GRAPH ONLY soft key to print out the absorption spectrum. Do not print out the table, as it takes a long time and includes several pages.
- Don't forget to exit the program to the main menu by pressing exit TWICE and then YES to the question, "Return to the Main Menu?"!
- Pour your undiluted DCPIP back into the test tube labeled DCPIP. You will need it in the next part of today's activities. You may discard the used plastic cuvettes in the trash can.
IF you are using the DU720 instrument follow these instructions:
Use of the Beckman DU®730 Spectrophotometer for Wavelength Scans (e.g., Absorption Spectra)
- In the main menu, choose wavelength scan
- Wavelength scan (350nm - 700nm). (Make sure that the y axis is showing Abs (absorbance) rather than % transmittance.)
- Put in cuvette with water as your instrument blank. Make sure that the clear side of your cuvette is directed so that the clear side is facing the light path (back/front) for this spec and not right/left as you would if using the DU530 specs.
- Press Blank
- When the blanking is complete put in cuvette with sample. Make sure that the clear side of your cuvette is directed facing the light path.
- Press Read
- When scan is complete use the two arrows found to the right of the display to find the peak absorbance wavelength and the absorbance at that wavelength. Record these data in your lab notebook.
To print:
- Press Options
- Press More
- Press Send Data
- Unclick Table
- Press OK
- Don't forget to exit the program to the main menu.
- Pour the undiluted DCPIP back into the test tube labeled DCPIP. You will need it in the next part of today's activities. You may discard the used plastic cuvettes in the trash can.
Directions for using the Beckman Spectrophotometers are also found in Appendix C.
Determining Concentration from Absorbance
B. Prepare Serial Dilutions
Each pair will use a stock solution (concentration 0.05mM) of DCPIP to prepare a series of 4 dilutions. You will then construct a standard curve by plotting absorbance (y) versus concentration (x) and calculate the concentration of an unknown from its absorbance.
- Label three 13mm test tubes with the dilution ratios: 1/2, 1/4, 1/8.
- With a 5.0mL serological pipette add 4.0mL distilled water to each tube.
- Using a clean pipette, add 4.0mL of the stock solution of DCPIP to the tube labeled 1/2 and mix by inversion (not vortexing). Use a square of Parafilm® to cover the test tube opening. Keeping the thumb on Parafilm®, invert the test tube twice.
- Use a clean pipette to add 4.0mL of the 1/2 dilution to the tube labeled 1/4; mix.
- Use a clean pipette to add 4.0mL of the 1/4 dilution to the tube labeled 1/8; mix.
- Calculate the final concentration of each dilution using the stock concentration of 0.05mM. Record the concentration of each of your dilutions in mM and in μM in your lab notebook. (You will use these final concentrations as points on the x axis of the standard curve that you will create.)
C. Determination of Absorbance
Use of the Spectronic20+ Spectrophotometer
You will find a different spectrophotometer at the end of your lab bench. This Spec20+ is used to measure absorbance at a set wavelength. NOTE that the Spec 20 instruments does NOT use square cuvettes!!! This instrument uses the round bottom disposable 13mm glass test tubes that you used when you made your dilutions.
An animation of how to use a Spec 20+ can be found at: | Spec20 Instructions
A diagram and general instructions for use of the Spectronic20+® (Spec20, informally) are provided. Please read the instructions before beginning the laboratory exercises. The meter on the Spec20 has 2 scales: a logarithmic scale for absorbance (A) and a linear one for percent transmittance (%T). We will use the %T scale to zero and to blank the instrument. We will use the A scale to take absorbance readings. Your instructor is available to answer any questions regarding the use of the spectrophotometer.

Figure 4. A Diagram of the Spectronic20+ Spectrophotometer.
- Your instructor has already turned on you instrument and let it warm up for at least 30min. When you are finished your lab work today, you will turn it off using the On-Off Knob (A).
- Set the wavelength using Wavelength Selector (D) to the wavelength that you determined as peak with your absorbance spectrum scan.
- Insure that the filter lever located at bottom front of instrument is in correct position for the wavelength you set (to the left for wavelengths 340-599nm; to the right for wavelengths 600-950nm).
- Use Zero-Adjust Knob (A) to set meter to 0%T.
- Wipe the outside of a 13mm glass test tube that is at least 2/3 full of water (blank solution) with a Kimwipe™, insert it into the Tube Holder (C), and close the lid.
- Use 100% Adjust Knob (B) to set meter to 100%T, which is equivalent to zero absorbance on the absorbance scale. Remove the blank tube.
- Wipe the outside of a 13mm glass test tube that contains undiluted stock DCPIP (tube must be ~2/3 full---if it isn't, add more), insert the test tube (NOT a plastic cuvette!!) into the Tube Holder (C), and close the lid.
- Record the absorbance reading from the absorbance scale (NOT the transmittance scale!), and remove the tube. Is the reading the same or close to the same Absorbance that you obtained from the Beckman spec at this wavelength for undiluted stock DCPIP?
- Repeat steps 7-8 for each of your dilutions of DCPIP.
- Record all the absorbance values in your lab notebook.
The directions for using the SPEC 20 is also found in Appendix B.
D. Generation of a Standard Curve by Linear Regression Analysis and Determination of the Concentration of a Solution with an Unknown Concentration
(The directions below for using Excel to create a scatter plat and linear regression analysis are also found in Appendix D and Appendix E )
Plot absorbance on the y axis and final concentration (NOT dilution!) on the x axis to generate a simple scatter plot. Then add a trendline, find the equation for the line, and assess its fit with your data.
Microsoft Excel Instructions for Scatter Plot and Linear Regression Analysis
- To launch Excel, click on the icon in the dock at the bottom of the screen. An Excel Workbook will open. If Microsoft Excel is already open, select New Workbook from the File menu to display a new spreadsheet.
- Assign a title to column A (the x-axis) of spreadsheet and enter concentration values in ascending order. Assign a title to column B (the y-axis) and record the measured absorbance values that correspond to each concentration.
- To select data to be plotted, highlight both columns, including headers.
- Click on the Charts tab below the toolbar. A gallery of chart types will appear below. Press the X Y (SCATTER) button on the far right to display the appropriate charts in this so-called Elements Gallery.
- To select the proper chart type from the Elements Gallery, press the “Straight Marked Scatter” thumbnail (second from the right)! A chart will appear on the worksheet with adjacent data points connected by straight lines.
- Open the Formatting Palette by pressing on the Toolbox icon. Under Chart Options you should label the axes and delete the title the Excel puts at the top of the graph. (Remember that in science writing graphs are figures and figure titles go below the graph just after the figure number.) Be sure to label the axes and give concentration units (mM or μM). There are no units for Absorbance because A is a unitless measurement. However, you should label the Y axis as Absorbance and include the wavelength in nm. You can also show or hide the gridlines in Chart Options.
- The chart may be created as an object in the worksheet, or it may be displayed as a new sheet. Under the Chart menu select Move Chart... to choose between these two options. Choose Display as New Sheet.
- Double-clicking on either axis will allow you to change the scale, font and other features of the axis.
If the data show a proportional relationship, fit the data to a line using linear regression. Linear Regression is a method of data evaluation that enables us to create a plot of absorbance data versus concentration for a series of dilutions. The plot generated by the linear regression method is called a standard curve, and is described by the equation y = mx + b, where m is the slope of the line, b is the y intercept, and x, y are the data points. Excel will provide an equation using y=mx +b for the line created.
Making a Trendline through Linear Regression Analysis
- Click on a data point on your graph to highlight all the data points. Under the Chart menu, select Add Trendline... The Format Trendline window will open.
- Press Type in the left column to select linear regression. Press Options in the left column to display the regression equation and the R-squared value on the chart.
- Analyze the R-square value (correlation co-efficient) . A correlation coefficient is a measurement of how well the regression line fits the actual data points (R2). An R2 value equal to 1 is indicative of perfect correlation among all the data points, and R2 values of 0.99-0.97 indicate good linearity. If acceptable linearity is demonstrated, the regression equation for your trendline can be used to calculate the concentrations of a solution by substituting the absorbance of the solution into the equation as the y value and solving for x.
If your standard curve is not linear, consider the types of errors that may have contributed to this lack of linearity.
When you are ready to print your graph, from the File menu select Page Setup... Choose the Orientation of the printout (portrait or landscape). Adjust the Scaling to a figure other than 100%, if desired. A 50% scaling works well for inserting graphs into your lab notebook.
The printer closest to L310 is called "Hallway HP4200" and is located outside the lab (L-310). Students in other rooms may use another printer closer to their lab. Ask your instructor about the location of the printers.
E. Determination of Concentration in an Unknown using a Standard Curve
There is an animation showing how to determine the concentration of a solution for which you have a standard curve at | http://www.wellesley.edu/Biology/Concepts/Html/standardcurve.html. Your lab instructor will give you a test tube with an unknown concentration of DCPIP. At the appropriate wavelength, measure the absorbance and determine the concentration of this solution using your the linear regression equation established from you standard curve. Before you leave the lab, please hand in your standard curve along with the calculated concentration of the unknown in both mM and μM units. Show all work!
Is there a clear relationship between the absorbance values and the concentrations of the four dilutions of Sample? Does this relationship follow the Beer-Lambert Law?
Laboratory Cleanup
- Place glass pipettes tips down in pipette canister.
- Discard Pasteur pipettes in red sharps container.
- Discard glass test tubes (disposable) in glass discard box in front of lab.
Part 2: Taster Data Analysis & Science Writing Workshop
In the second part of lab, you will participate in a peer review of the drafts of the figures and a results and discussion section that you prepared for homework. Your instructor will conduct a writing workshop and discuss expectations for the scientific research report that you will write on the Taster PTC experiment.
Assignment
Turn in at the beginning of Lab 9
1. (35 points) Your completed TASTER paper is due at the beginning of Lab 9. Please write a partial scientific paper (Title, Abstract, Results, Discussion, and References). Your instructor will provide more detailed instructions during your writing workshop. This assignment must be done individually.
2. In your lab notebook, prepare a flow chart for the Hill Reaction (Lab 9).
3. Review the mechanisms of electron transport and proton flow in photosynthesis in your textbook.
